The name of this compound is:
CO
What is carbon monoxide?
A solution with a pH = 8.4 is acidic, basic, or neutral?
What is basic?
This state of matter has definite volume but no definite shape.
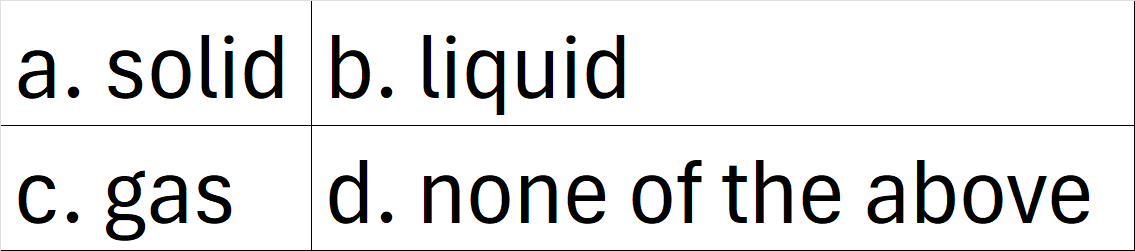
What is b. liquid?
The molecular geometry of this molecule is
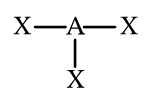
What is trigonal planar?
The molar mass of Ca3(PO4)2 is
What is 310.18 g/mol?
The chemical formula for this compound is:
sodium hydroxide
What is NaOH?
Is Ag2S soluble or insoluble in water?
What is insoluble?
Transferring of one or more electrons between a metal and a nonmetal forms this type of bond.
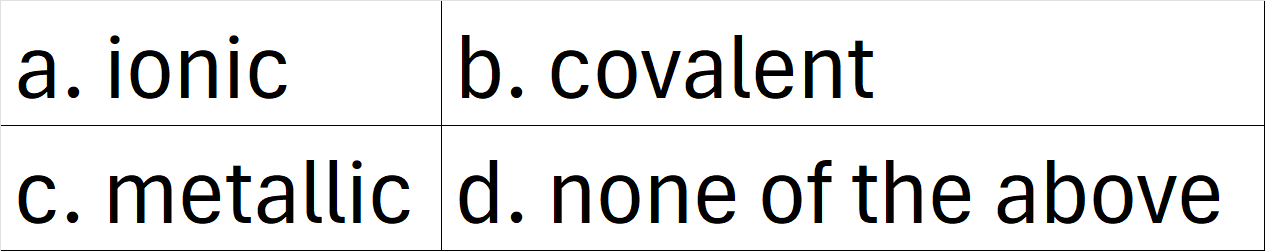
What is a. ionic?
The molecular geometry of this molecule is

What is bent?
Complete the table below.
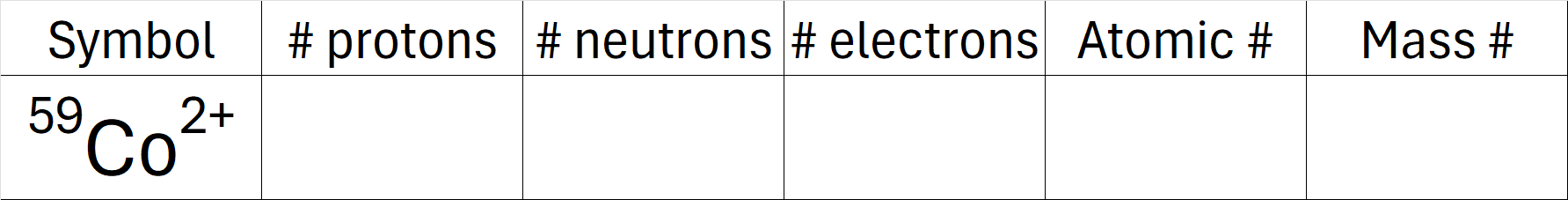
What is
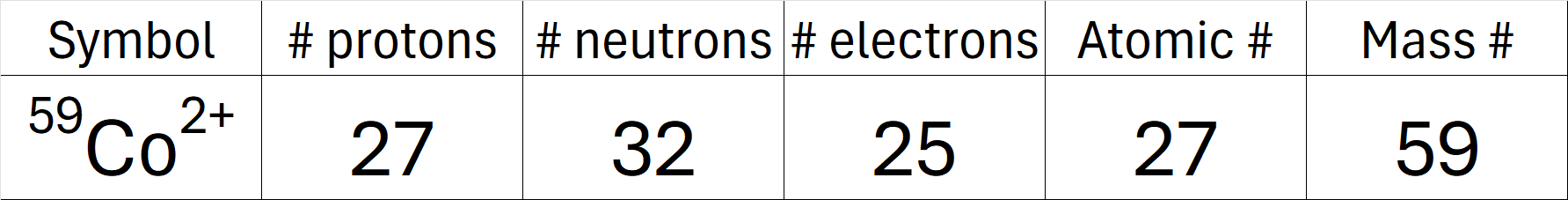
The chemical formula for this compound is:
potassium nitride
What is K3N?
All atoms/molecules/compounds exhibit these types of intermolecular forces.
What are c. dispersion forces?
This type of reaction results in the formation of multiple products from a single reactant.

What is d. decomposition?
What is the polarity of all trigonal pyramidal molecules?
What is polar?
When [H3O+] = 6.8 x 10-8 M, [OH-] is equal to this
What is 1.5 x 10-7 M?
The name of this compound is:
HNO3
What is nitric acid?
When the following equation is balanced, the coefficient on oxygen is
_C2H2 (l)+_O2 (g)->_CO2 (g)+_H2O (g)
What is 5?
When a reactant loses electrons during a chemical process, it is said to undergo this.

What is a. oxidation?
The electron group geometry of this molecule is
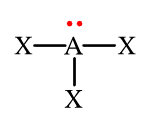
What is tetrahedral?
The volume (mL) of a 6.75 M BaCl2 solution required to prepare 420 mL of a 2.25 M BaCl2 solution is
What is 140 mL?
The name of this compound is:
FeSO4
What is iron (II) sulfate?
According to LeChatelier's principle, increasing the concentration of a product, shifts the equilibrium in this direction.
What is b. towards reactants?
This type of reaction requires a net absorption of heat, meaning heat is a reactant.

What is b. endothermic?
Linear molecules are polar when...
What is the outer two atoms are different?
The molarity of a solution that contains 245 g of NaNO3 in 2.00 L of solution is
What is 1.44 M?