Ionic compounds are formed by bonds between these types of elements.
What are metals and nonmetals?
Covalent molecules are formed by bonds between this type of element.
What are nonmetals?
The Lewis dot notation for the element sulfur shows this many valence electrons.
What is 6?
VSEPR theory is a model for predicting this about a molecule.
What is shape or geometry?
If atoms that share electrons have an unequal attraction for the electrons, the bond is called this.
What is polar?
What is 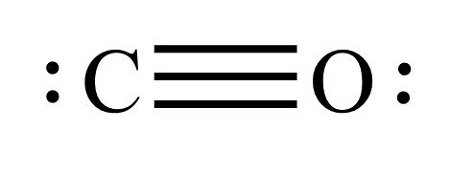 ?
?
In an ionic bond, the electrons are ______ between atoms.
What is transferred?
In a covalent bond, the electrons are _______ between atoms.
What is shared?
The Lewis structure for the ionic compound HCl is this.
What is 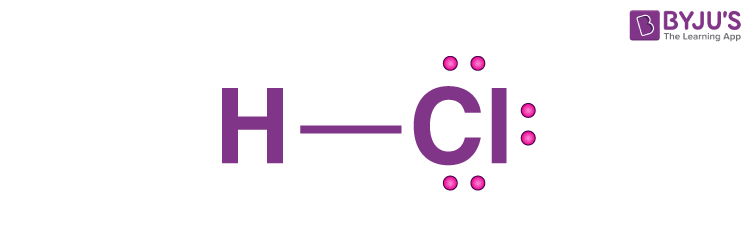 ?
?
When determining the geometry around a central atom, double and triple bonds count as this many bonds.
What is one?
What is nonpolar?
The ions in most ionic compounds are organized into a fixed structure called this.
This is the amount of shared electrons that a double bond represents.
The central atom in a Lewis structure is generally the one with the _________ electronegativity.
What is least?
This is shape of the nitrate ion, NO3-.
What is trigonal planar.
This type of intermolecular force is the strongest.
What are hydrogen bonds?
Properties of ionic compounds include high melting point and this.
What is hardness/brittleness/solubility?
The electrons that are shared between atoms in covalent bonds are known as this.
The Lewis structure for NH2Cl is this.
What is
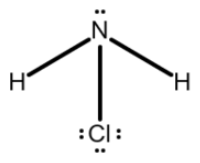
?
This is the shape of NH2Cl.
This is the name for the partially positive and partially negative areas of a polar bond.
What are dipoles?
Ionic compounds have higher melting points than molecular compounds because they have a greater ________.
What is attractive force between atoms?
In a covalent bond, the atom that attracts electrons more strongly has a larger value of this property.
What is electronegativity?
The Lewis structure for ONCl is this.
What is
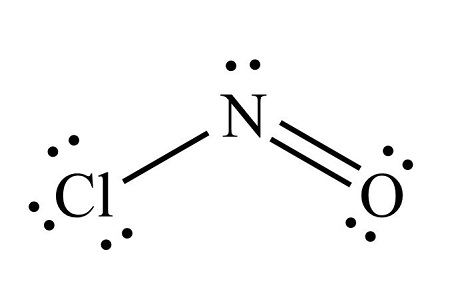
?
This is the shape of ONCl.
What is bent?
Polarity is determined by electronegativity difference and this.
What is shape/geometry?