What charge does an electron have?
How many protons does this elements have?
7
B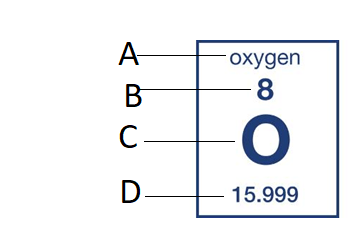
What is the Atomic Number?
Monosaccharides are the subunit of which biomolecule?
What is carbohydrates?
All organic (living) compounds contain which element?
What is carbon?
Determines the element.
What are protons?
How many neutrons does this element have?
What is 8?
C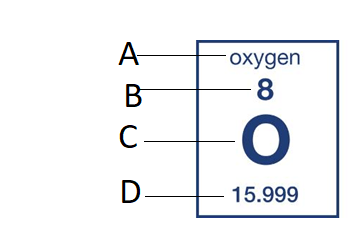
What is the symbol?
Which biomolecule’s main function is to store genetic information in the nucleus of cells?
What is Nucleic Acids?
Examples are RNA and DNA.
What is nucleic acids?
Particle(s) are found in the nucleus.
What are protons and neutrons?
If an element’s atomic # is 6, has 6 neutrons, and has 7 electrons what is this elements atomic mass?
What is 12?
D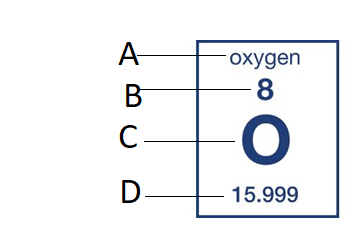
What is the atomic mass?
The 2 subunits of lipids.
What are fatty acids and glycerol?
Elements are present in ALL biomolecules.
What are carbon, hydrogen, and oxygen?
The number of electrons that can fit in the 2nd energy level.
What are 8 electrons?
If Carbon 14 has a mass of 14 and an atomic number of 6. How many neutrons are in this particular type of carbon atom?
What are 8 neutrons?
Which element has an atomic # of 16 and atomic mass of 32?
What is Sulfur?
Provides energy and support for plant cells(in cell walls) and also provides carbon for biosynthesis?
What are carbohydrates?
Elements that make up nucleic acids.
What are Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, and somtimes Sulfur.
How many more electrons could fit in this outermost shell?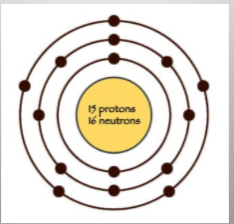
What is 13?
How many valence electrons would this element have?
What are 6 valence electrons?
Which element is this?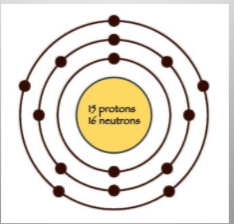
What is phosphorus?
Provides energy and makes up most of the cell membrane of all cells.
What are lipids?
An example is an enzyme.
What are proteins?