True or False: CH4 is a polar molecule
False
Is ammonia gas (NH3) more soluble in water (H2O) or methane (CH4)? Why?
Water. "like dissolves like" - both polar
True or False: Light is emitted when electrons get excited and light is absorbed when electrons return to the ground state.
False (other way around!)
Is the following reaction endothermic or exothermic?
CH4(g)+2O2(g)→CO2(g)+2H2O(l) ΔH=–890kJ
Exothermic
Specific heat capacity of water (including unit)
4.18 Jg-1k-1 or Jg-1oC-1
What shape is CO2?
Linear
What intermolecular forces are present in Propan-2-one?
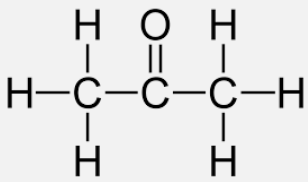
Dispersion forces and Dipole-dipole forces
What is this apparatus used for?
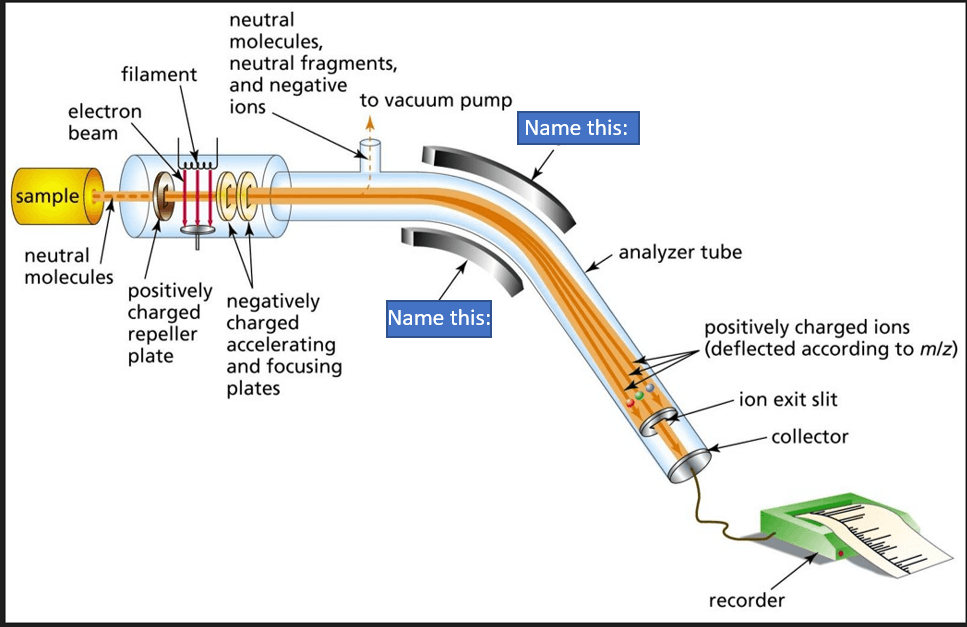
Determination of mass
A student places a strip of magnesium into hydrochloric acid. The student touches the test tube and notices that it feels hot. Is this reaction exothermic or endothermic?
Exothermic
Identify each variable in the equation (including unit):
q=mcΔT
q - Thermal energy (J or kJ)
m - mass (g)
c - Specific heat capacity (Jg-1k-1 or Jg-1oC-1)
ΔT - Change in temp (Tf-Ti) (K or oC)
How many areas of electron density are there in H2O?
4
What are 2 secondary bonds that can affect boiling point of a substance?
Dispersion forces, dipole-dipole interactions, hydrogen bonding
The thin layer chromatography plate shown below has a polar stationary phase. It was developed using hexane (C6H14) as the solvent.
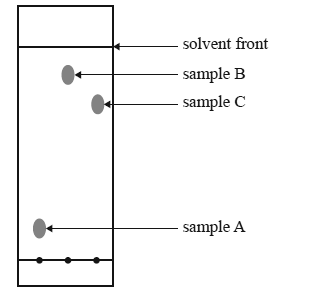 Which sample has the most polar molecules?
Which sample has the most polar molecules?
Sample A
The diagram below shows an exothermic or endothermic reaction. Which reaction is shown?
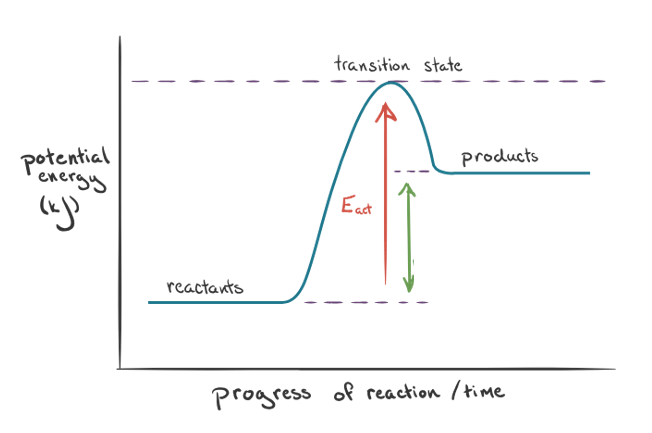
Endothermic
40mL of water is heated from 22oC to 50oC. Calculate the thermal energy used to heat the water in kJ (2 sig figs)
q= 4.7 kJ (q = 4,6816 J)
What is the shape of NCl3
Trigonal Pyramidal
Draw the lewis structure of Phosphorus trichloride

Calculate the retention factor (Rf) of Analyte (A) in the following chromatogram:
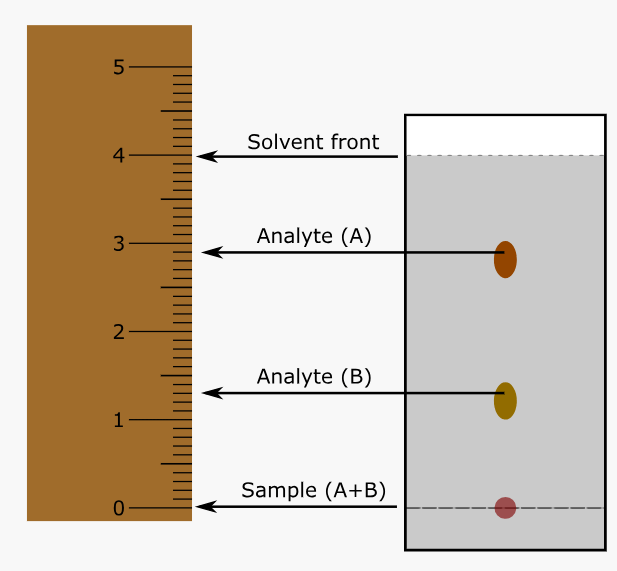
0.725 (0.75 if used 3)
Calculate the enthalpy change of the following reaction:
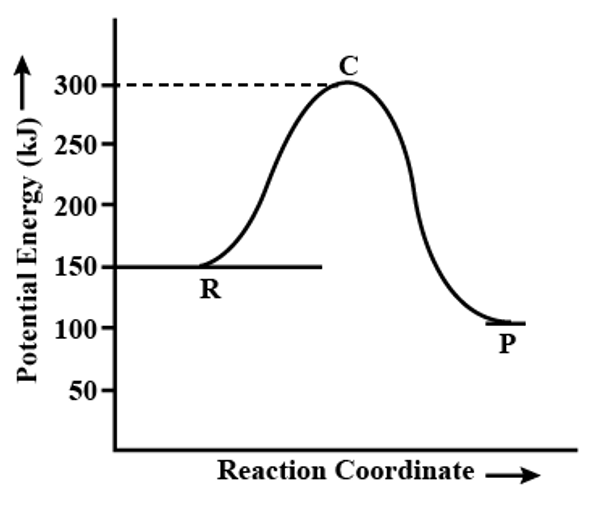
-50 kJ
A 0.850g sample of fuel was burnt under a steel can containing 150mL of water. After the flame went out, the mass of the fuel was 0.300g and the temperature of the water had risen by 22.5 °C. Calculate the energy content of the fuel in kJg-1
26 kJg-1
What is the shape of SiFCl3?
Tetrahedral
List the following molecules in order of increasing melting points: C3H8, CH4, CH3COOH, C2H6
Higher melting points corresponds to stronger intermolecular forces. Go through the list above.
1. CH3COOH has the ability to hydrogen-bond. It will have the strongest intermolecular forces.
2. Of the molecules that are left, the largest one (C3H8) likely has the strongest dispersion forces, as dispersion forces increase as molar mass increases. The smallest (CH4) likely has the weakest intermolecular forces.
The answer is: CH4, C2H6, C3H8, CH3COOH
Calculate the relative atomic mass of the following element:
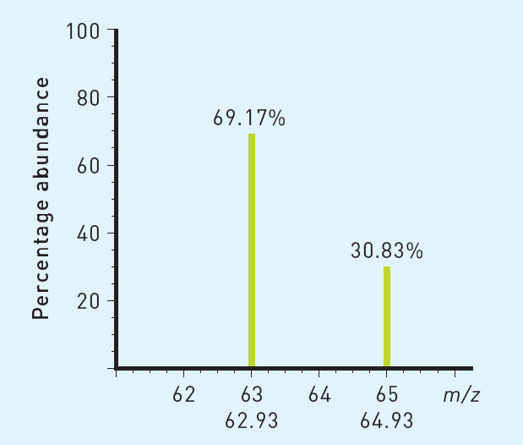
63.54
Methane is burnt in Oxygen to produce Carbon Dioxide and water vapor. Calculate the approximate enthalpy change for this reaction using bond enthalpy changes:
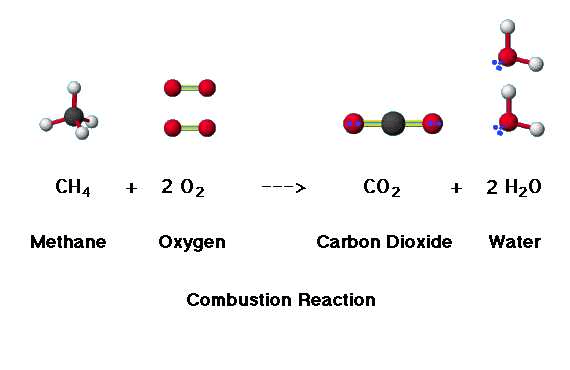
-808 kJ mol-1
19.44g of Magnesium reacted with HCl to produce 5.9kJ of heat. Determine the molar enthalpy of the reaction if the magnesium is the limiting reagent.
7.38kJ