What is the percent by mass of Na in sodium carbonate?
43.4%
Rank these compounds in order of increasing boiling point: ethane (CH3CH3) formaldehyde (CH2O) hydrogen peroxide (H2O2)
(a) CH3CH3 < CH2O < H2O2
The heating curve below shows how the temperature of a substance changes as heat is added. What represents the heat involved in going from point D to point E?
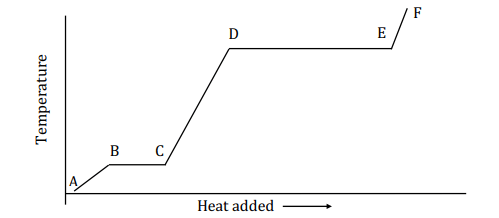 (a) mC∆T (b) ∆Hfus (c) ∆Hsublimation (d) ∆Hvap (e) ∆H0 f
(a) mC∆T (b) ∆Hfus (c) ∆Hsublimation (d) ∆Hvap (e) ∆H0 f
(d) ∆Hvap
How many electrons in total can be contained in any p subshell?
6
Which molecule has the correct molecular geometry listed?
(a) BH3 – bent
(b) CH4 – square planar
(c) SF4 – tetrahedral
(d) PF5 – trigonal bipyramidal
(e) H2S – linear
(d) PF5 – trigonal bipyramidal
When 1 mol magnesium chloride dissolves in water and dissociates, how many moles of ions are produced?
3 moles
Identify the precipitate that forms when an aqueous solution of barium chloride is mixed with an aqueous solution of sodium sulfate.
(a) BaSO4 (b) Na2SO4 (c) NaCl (d) BaCl2 (e) There would be no precipitate
(a) BaSO4
On the phase diagram below, what phase(s) would be present at the point “A?”
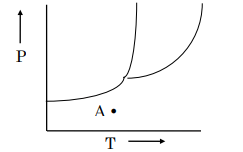
Gas
Which atom corresponds to the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 ?
Mn
How many protons, neutrons, and electrons are in the chloride ion isotope 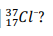
17 p, 20 n, 18 e
How many moles are in 12.0 g of P atoms?
0.387 mol
What volume of 0.200 M NaOH would it take to react exactly with 25.0 mL of 0.100 M H2S, according to: H2S (aq) + 2 NaOH (aq) → Na2S (aq) + 2 H2O (l) ?
25.0 mL
Calculate the frequency of red light with a wavelength of 650 nm.
4.61 × 1014 Hz
Rank the elements P, Si and N in order of increasing atomic radius.
N < P < Si
In the nitrate ion, NO3– , what hybrid orbitals are used by nitrogen in bonding?
sp2
For the reaction 2 KClO3 → 2 KCl + 3 O2, how many molecules of oxygen are produced when 0.82 moles of potassium chlorate decompose?
7.4 × 1023
Al (s) reacts with water to form aqueous aluminum hydroxide and hydrogen gas. Write a balanced equation using the smallest whole numbers as coefficients. What is the coefficient of H2O?
6
466 g of water initially at 74.6 °C releases 129 kJ of heat as it cools. What would be the final temperature of the water? The specific heat capacity of water is 4.18 J/g.°C
8.4 ºC
Which of these is not a valid Lewis structure for sulfur trioxide?
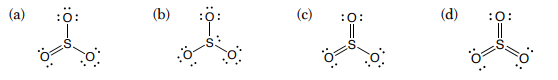
B
Name this compound: Pb(CO3)2
lead(IV) carbonate
The density of bromine liquid is 3.12 g/mL. Since it is a liquid, it is easier to measure in a graduated cylinder than to weigh out on a balance. If we needed 28.1 g of Br2 for a reaction, what volume would we measure out? (a) 87.7 mL (b) 9.01 mL (c) 25.0 mL (d) 0.111 mL (e) 0.549 mL
9.01 mL
What is the volume in liters occupied by 7.40 g of carbon dioxide gas at 25 °C and 1.09 atm pressure?
3.77 L
How much heat would be released if 36 g of methane (molar mass: 16.05 g/mol) burned according to this thermochemical equation?
CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l)
∆Hrxn = –891 kJ
2.0 × 103 kJ
The formal charge on the phosphorus in the Lewis structure shown of the phosphate ion (PO4 3– ) is:
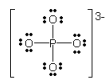
+1
Iron(II) nitrate [Fe(NO3)2 (aq)] reacts with aqueous potassium hydroxide [KOH (aq)]. Write the net ionic equation for this reaction.
Fe2+ (aq) + 2 OH– (aq) → Fe(OH)2 (s)