Which property of water allows it to move upward from the roots of a tree to its leaves?
A. High specific heat
B. High heat of vaporization
C. Cohesion
D. Solvent of life
The correct answer is (C). As water evaporates from the leaves of trees, the property of cohesion (attraction to another water molecule) allows the water molecules leaving the plant to pull molecules upward because they are attracted though hydrogen bonds.
Which type of chemical bond involves the transfer of electrons from one atom to another, resulting in oppositely charged ions that are attracted to each other?
A. Covalent bond
B. Ionic bond
C. Hydrogen bond
D. Van der Waals interactions
The correct answer is (B). Covalent bonds involve the sharing of electrons. Ionic bonds involve the transfer of electrons, causing the atoms to have opposite charges and attract each other. Hydrogen bonds result from the attraction between partial positive and negative charges in atoms. Van der Waals interactions are weak attractions that occur when atoms and molecules are very close together.
What elements make up nucleic acids?
What is carbon, hydrogen, oxygen, nitrogen, phosphorous
All of the following statements are true about lipids, EXCEPT that _____.
all lipids are fats
lipids are biological molecules that are insoluble in water
lipids are good at storing energy
steroid hormones like testosterone are lipids
All lipids are fats
There are four levels of protein structure. Which level includes the coils of the α helix and the folds of the β pleated sheets?
A. Primary structure
B. Secondary structure
C. Tertiary structure
D. Quaternary structure
The correct answer is (B). Primary structure refers to the sequence of amino acids. Secondary structure includes the α helix and the β pleated sheets. Tertiary structure is the overall shape of the protein. Quaternary structure consists of two or more polypeptide chains aggregated into a functional macromolecule.
There are several emergent properties of water that contribute its powerful affect on living organisms. These emerging properties are mostly due to:
A. The abundance of water on Earth
B. The hydrogen bonds linking water molecules together
C. Water’s ability to moderate pH
D. The buffering ability of water
The correct answer is (B). The hydrogen bonds between water molecules are able to form, break and re-form frequently. Water’s life-sustaining properties are a result of hydrogen bonding that orders molecules into a higher level of structural organization.
The repeating units in both DNA and RNA are called?
- nucleotides
Which of the following molecules is a carbohydrate?
A. Palmitic acid
B. Collagen
C. Insulin
D. Lactose
The correct answer is (D). Anything that ends in the suffix –ose is a sugar and sugars are carbohydrates.
Which of the following statements correctly describes the difference between saturated and unsaturated fats?
A. Saturated fats tend to be solid at room temperature; unsaturated fats are liquid at room temperature
B. Saturated fats are cannot pack together closely because of their bent structure; unsaturated fats can pack together because they are flat
C. Saturated fats come from plants and fish; unsaturated fats are from animals (except fish)
D. Saturated fats are considered “healthy” fats; unsaturated fats may contribute to cardiovascular disease
The correct answer is (A). Saturated fats tend to be solid at room temperature and are mostly obtained from animal, not plant, sources. Unsaturated fats are liquid at room temperature and are primarily obtained from plants and fish sources. Healthful diets tend to be higher in unsaturated fats and lower in saturated fats.
What determines the function of a protein?
A. The number of peptide bonds
B. The types of side chains
C. The protein’s specific structure
D. The tissue that produces the protein
The correct answer is (C). The function of a protein depends on its ability to recognize and bind to some other molecule. For example, antibodies are specific to the invading virus or bacteria, and enzymes are specific to a substrate.
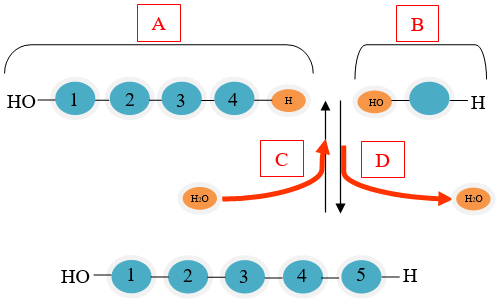
Which part of the diagram is showing a monomer?
A
B
C
D
The correct answer is (B). Monomers are small units that are joined together to form polymers.
What are the four nitrogenous bases and how do they compliment each other ?
Adenine to Thymine
Cytosine to Guanine
What elements make up carbohydrates?
answer choices
1.Carbon, hydrogen, nitrogen, oxygen
2.sulfur, carbon, hydrogen
3.glucose and fructose
4.carbon, hydrogen, oxygen
carbon, hydrogen, oxygen
Fatty acids with hydrogen atoms artificially added to the double bond is called...
Monounsaturated fatty acids
Trans fatty acids
unsaturated fatty acids
Saturated fatty acids
Trans fat
What is the monomer (building block) of a protein?
AMINO ACID

Which part of the diagram shows a condensation reaction?
A
B
C
D
The correct answer is (D). Condensation reactions occur when monomers are connected by the removal of a water molecule.
What makes up the backbone of DNA?
A phosphate and Pentose sugar (Deoxy Ribose Sugar) linked in repeating units
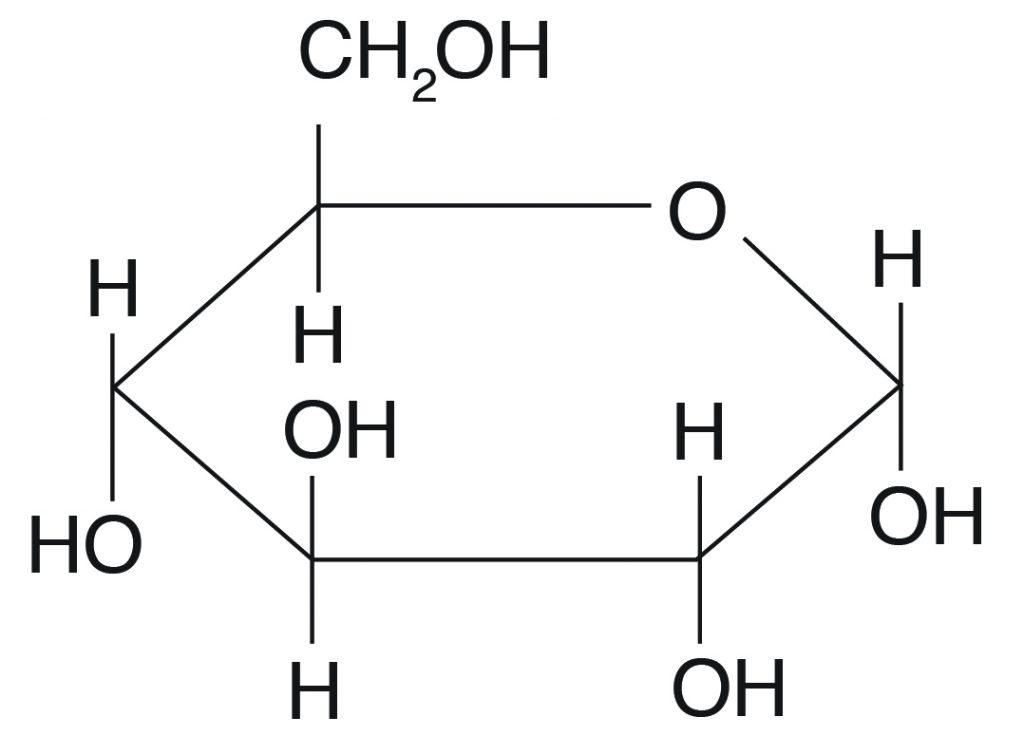
Q. What type of carbohydrate is this?
polysaccharide
disaccharide
monosaccharide
it isn't a carbohydrate
Polysaccharide
What are different examples of lipids?
answer choices
1.monosaccahrides, disaccharides, starch, cellulose
2.dipetide, polypeptide, amino acids
3.DNA, RNA, ATP
4.triglycerides, waxes, steroids, phospholipds
4.triglycerides, waxes, steroids, phospholipds
Which of the following is a protein (amino acid)?
A
B
C
D
A. R group is a dead give away
What are the main three differences between DNA and RNA?
RNA uses Uracil
RNA is a single strand
RNA has a Ribose sugar
A Triglyceride is composed of:
answer choices
Glycerol, 2 fatty acids
3 Glycerols and 1 fatty acids
3 Glycerides
Glycerol, 3 fatty acids
Glycerol, 2 fatty acids
Identify the element found in a protein that is not present in a carbohydrate
Carbon Oxygen Hydrogen Nitrogen
Nitrogen found in the amino group amino group

