Where is the atomic number located?
Above the element symbol.
How many valence electrons does Carbon have?
4
Where are metals located on the periodic table?
Its located on the left of the periodic table.
What are the four states of matter?
Solid, liquid, gas and plasma.
What are the pH range for acids?
0-6
How many protons, neutrons and electrons does the element Neon have?
Proton:10 Neutron:10 Electron:10
What group is the most reactive?
Alkali metals.
Is 2NH2=2N2H balanced or unbalanced?
Its unbalanced
What does a solid become after it melts?
It becomes liquid.
What are the pH range for bases?
8-14
What is the Lewis dot structure for Calcium?

What is one property of an ionic bond?
They have high melting points and high boiling points or they have high melting points and high boiling points or they're hard and brittle or they conduct electricity when they are dissolved in water or they're good insulators.
What does the law conservation of matter state?
Matter cannot be created or destroyed.
What does Boyle's law state
The pressure exerted by a gas is inversely proportional to the volume occupied by it.
What affects the rate of solubility?
Stirring, mixing and breaking it into smaller pieces.
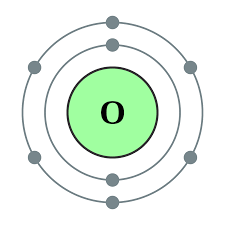
What is the bohr model for the element oxygen?
How is an ionic bond formed?
Its formed by the complete transfer of some electrons from one atom to another.
What are two properties of nonmetals?
They have high melting points and they are ductile or they are bad conductors of heat and electricity or they may be solids, liquids or gases at room temperature.
What does Charles's law state?
The volume of a gas equals a constant value multiplied by its temperature as measured on the kelvin scale.
What is a chemical change?
When one chemical substance is transformed into one or more different substances.