There are _____ minutes in 5 centuries
What is 3 xx 10^8?
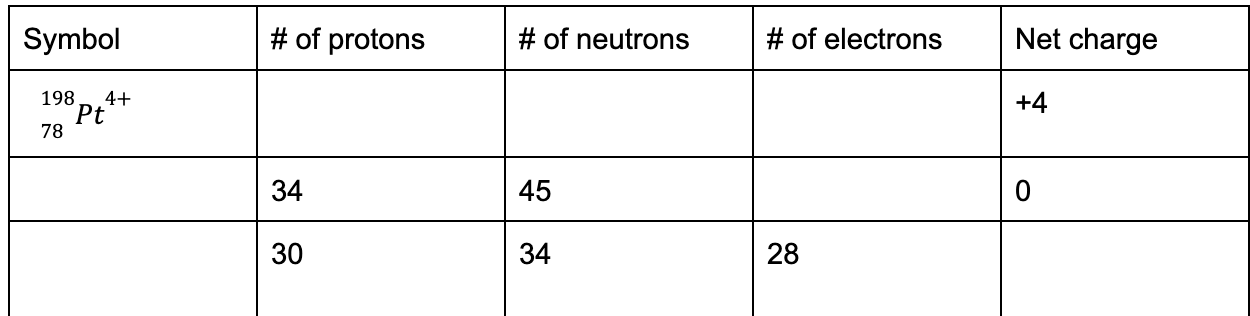
Isotopes vary in their number of this subatomic particle
What is neutrons
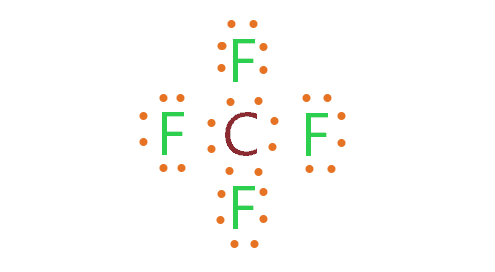
The type of bond type between carbon and fluorine atoms in the compound CF_4
What is covalent
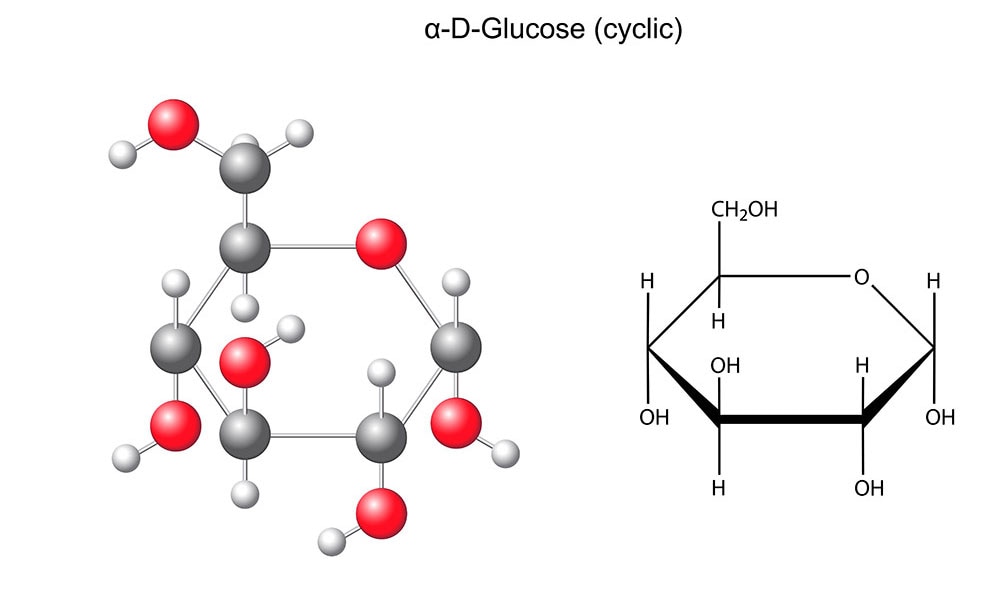
The empirical formula of glucose (molecular formula: C_6H_12O_6)
What is CH_2O_
The number of oxygen atoms in 2.75 moles of diatomic oxygen (O_2)
What is
3.31 xx 10^24
If a car has traveled 32.19 kilometers in 3.4 xx 10^6 milliseconds, its speed is ____ miles per hour.
Mile = 5280 feet
Inch = 2.54 centimeters
What is 21 miles per hour
One common property shared by the alkaline earth metals
What is having two lone valence electrons
OR
What is a charge of +2
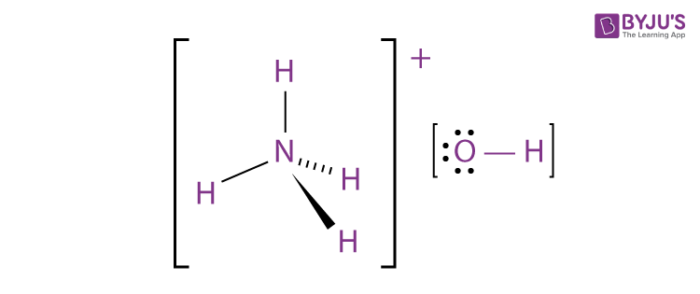
The bonds in NH_4OH are:
1. ______ bonds between nitrogen and hydrogen atoms
2. ______ bonds between nitrogen and oxygen atoms
3. ______ bonds between oxygen and hydrogen atoms
What is 1. covalent, 2. ionic, 3. covalent
The balanced version of the equation KClO_3 -> KCl + O_2
What is 2KClO_3 -> 2KCl + 3O_2
The mass of carbon in 0.500 mol of sucrose, C_12H_22O_11
What is 72.0 grams
At 25 degrees Celsius, when added to water (density: 0.997 g/mL), the organic solvent chloroform (density: 1.489 xx 10^-3 (mg)/(mm^3) g/mL) will settle ________ (above/below) the water.
What is below the water level
Chloroform has a density of 1.489 g/mL. This makes chloroform more dense than water, and so it will settle to the bottom

Daily double
The chemical formula for zinc carbonate
What is
ZnCO_3
The balanced version of the equation P_4O_10 + H_2O -> H_3PO_4
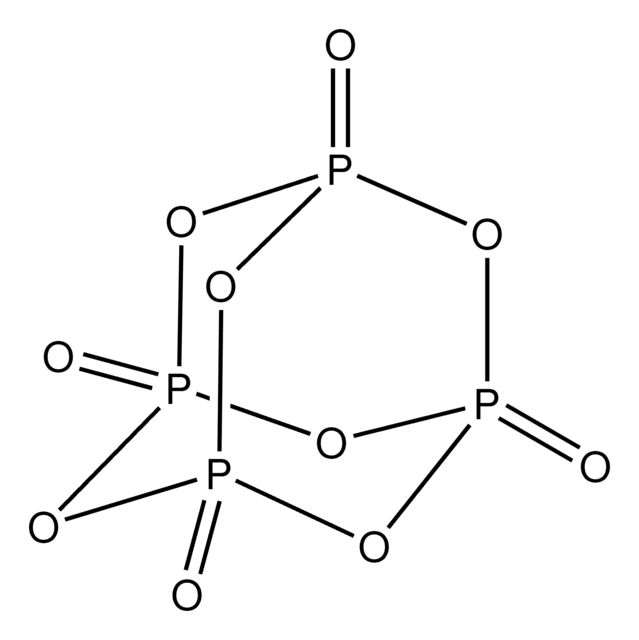
What is P_4O_10 + 6H_2O -> 4H_3PO_4
Mercury (II) oxide decomposes into liquid mercury and oxygen gas. _____ moles of oxygen gas result from the decomposition of 1.5 grams of mercury (II) oxide.

What is 3.5 xx 10^-3 moles of oxygen gas
The amount of volume in cubic meters that a perfect cube of 9.9 moles of Tungsten metal will take up, given that the density of Tungsten at 20 degrees Celsius is 19.254 g/cm3

What is 9.5 xx 10^-5 m^3 ?
A new element, Humboldtium, has been discovered, and 5 stable isotopes have been identified: Hu-299 (30.37% relative abundance), Hu-300 (50.05%), Hu-302 (11.12%), Hu-303 (7.80%), and Hu-305 (0.66%).
The atomic mass that should be reported on the periodic table is ____ (known to 2 decimal places)
What is 300.19 amu
The chemical formulas and charges for chlorite ion, perchlorate ion, chlorate ion, and hypochlorite ion, in this order.
What is
ClO_2^-, ClO_4^-, ClO_3^-, ClO^-
The balanced molecular equation for the combustion reaction undergone by acetone, C_3H_6O (l)

What is C_3H_6O (l) + 4O_2 (g) -> 3CO_2 (g) + 3 H_2O (g)
Ethane, C_2H_6, is undergoing a combustion reaction in a sealed container (yikes!). There are 11 grams of diatomic oxygen gas available while there are 5.0000 grams of ethane inside, so ______ is the limiting reagent (assuming the container doesn't explode).
What is oxygen gas (O_2)
The density ((g)/(cm^3))
of a rubber ball, given that it has a diameter of 6.350 cm and a mass of 0.1417 kg.
V_(sphere) =4/3pir^3
pi=3.1416
What is
1.057 g/(cm^3)
Antimony has two stable isotopes, Sb-121 (actual atomic mass: 120.90 amu) and Sb-123 (122.90 amu), and the atomic mass of the element is 121.76 amu.
The relative abundances of Sb-121 and Sb-123 are _____ and _____, respectively.
What is 57% and 43%
The chemical formula for nickel (II) phosphate

What is
Ni_3(PO_4)_2
Empirical formula of oxalic acid, given that the compound is 71.04% oxygen by mass, 26.68% carbon by mass, and 2.24% hydrogen by mass.
What is CHO_2

Copper (II) sulfate and sodium hydroxide react to form a precipitate of copper (II) hydroxide (molar mass: 97.561).
You add 1.8970 grams of copper (II) sulfate (molar mass: 159.609) to a solution containing 1.7556 grams of sodium hydroxide (molar mass: 39.997). If the reaction goes to completion and all of the limiting reactant is consumed, you will end up with _______ grams of copper (II) hydroxide.

What is 1.8966 g of copper (II) hydroxide