What is the purpose of gloves? (Unit 1 PAGE 4)
a. to protect your eyes b. to protect your ears
c. to protect your hands d. to protect your mouth
c. to protect your hands
# 1 WRITE DOWN:
Gloves protect your hands
Which state of matter has the most energy? (Unit 1 PAGE 18)
a. solid. b. liquid
c. gas d. plasma
c. gas
#6 WRITE DOWN:
Gas has the most kinetic energy.
The atomic number of an element is based on the number of: (Unit 1 PAGE 25)

a. Number of electrons b. Number of Protons
c. Number of neutrons d. Protons + Electrons
b. Number of Protons
#11 WRITE DOWN
Atomic number is the number of Protons
What is Electronegativity? (Unit 2 PAGE 2-3)
a. The distance between the nucleus and the final orbital.
b. How much energy it takes to remove an electron
c. How much an atom attracts electrons
d. 67
c. How much an atom attracts electrons
#16 WRITE DOWN
Electronegativity is how attractive an atom is to electrons.
O has bonded with a second O atom. Both atoms are sharing electrons. What type of bond is being described? (Unit 2 PAGE 8-9)
a. Ionic Bond. b. Covalent Bond
c. Metallic Bond d. Hydrogen Bond
b. Covalent Bond
#21 WRITE DOWN
Covalent bond shares electrons
Why is it important to wear safety goggles? (Unit 1 PAGE 4)
a. to protect your eyes b. to protect your ears
c. to protect your hands d. to protect your mouth
a. to protect your eyes
#2 WRITE DOWN:
Safety goggles protect your eyes
Which of the following molecules have low energy and is in a fixed position? (Unit 1 PAGE 18)
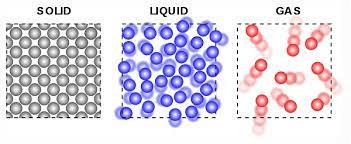
a. solid b. liquid c. gas
a. solid
# 7 WRITE DOWN:
Solids have the least amount of kinetic energy
What is inside the nucleus? (Atomic mass) (Unit 1 PAGE 25)
a. Electrons b. Protons + Electrons
c. Neutrons d. Neutrons + Protons
d. Neutrons + Protons
#12 WRITE DOWN
Atomic mass is made of Protons + Neutrons
Which of the following has the smallest atomic radius? (Unit 2 PAGE 2-3)
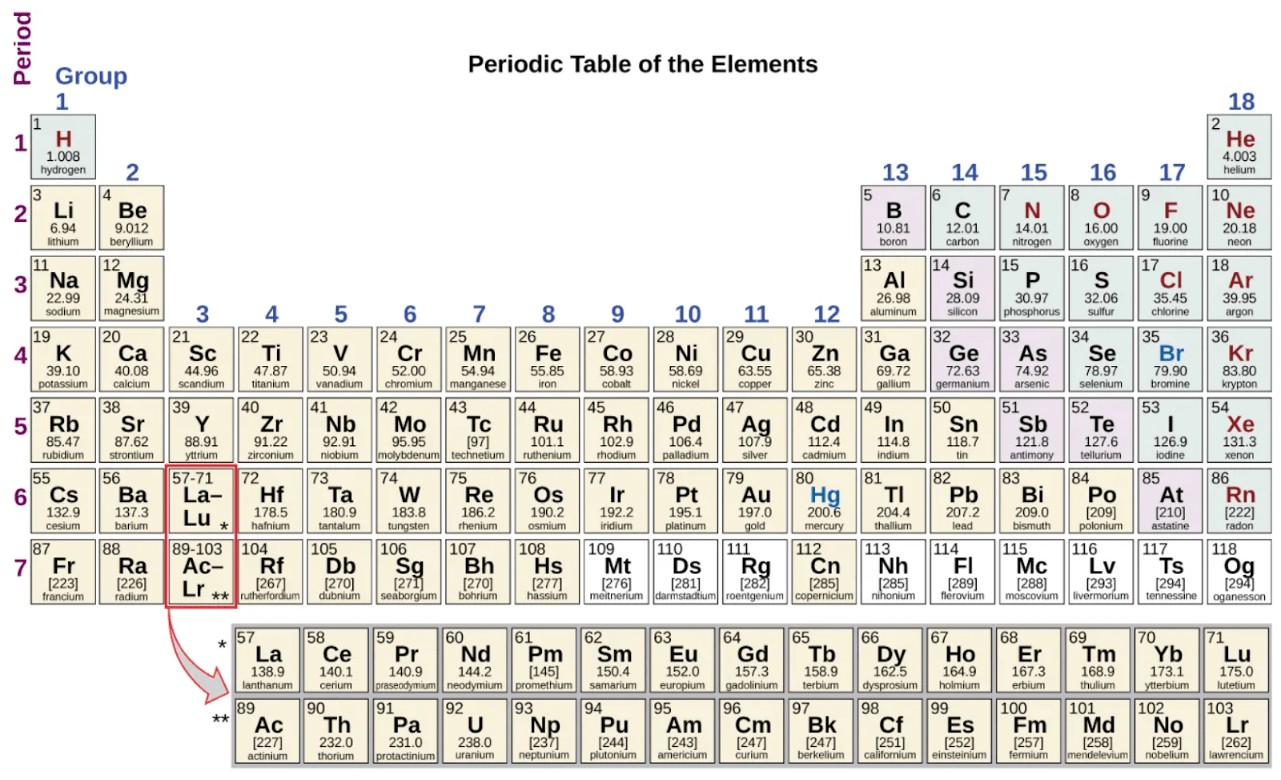
a. Cesium (Cs) b. Fluorine (F)
c. Chlorine (Cl) d. Francium (Fr)
b. Fluorine (F)
#17 WRITE DOWN:
Fluorine is the least right and down.
Na has bonded with Cl by giving Cl its valence electron. What type of bond is being described? (Unit 2 PAGE 8-9)
a. Ionic Bond b. Covalent Bond
c. Metallic Bond d. Hydrogen Bond
a. Ionic Bond
#22 WRITE DOWN
Ionic Bonds lose or gain electrons
A student wants to use a thermometer to measure volume. What should the student use? (Unit 1 PAGE 9)
a. A thermometer. b. A scale
c. A ruler d. A graduated cylinder
d. A graduated cylinder
#3 WRITE DOWN:
A graduated cylinder measures volume in liters
Which answer correctly shows the shape of each state of matter? (Unit 1 PAGE 18)
a. Liquids (indefinite form), solids (indefinite form), gas (indefinite form)
b. Liquids (definite form), solids (indefinite form), gas (definite form)
c. Liquids (indefinite form), solids (definite form), gas (indefinite form)
d. Liquids (definite form), solids (definite form), gas (definite form)
c. Liquids (indefinite form), solids (definite form), gas (indefinite form)
#8 WRITE DOWN:
Liquids - indefinite form, solids - definite form, gas - indefinite form
Element As has 33 protons and a mass number of 75. How many neutrons are in the nucleus of As? (Unit 1 PAGE 25)
a. 67 b. 42
c. 108 d. 48
b. 42
#13 WRITE DOWN:
75 mass - 33 protons = 42 neutrons
What is ionization energy? (Unit 2 PAGE 2-3)
a. The amount of energy to remove a proton
b. The amount of energy to remove a neutron
c. The amount of energy to remove an electron
d. How attractive an atom is to electrons
c. The amount of energy to remove an electron
#18 WRITE DOWN:
Ionization energy is the amount of energy to remove an electron
Element Rb (0.8) is bonding with Br (2.8) atoms to form RbBr. Calculate what type of bond was formed. (Unit 2 PAGE 9)
a. Nonpolar covalent bond b. Ionic bond
c. Polar covalent bond d. Hydrogen bond
b. Ionic bond
#23 WRITE DOWN:
2.8 - 0.8 = 2.0 and 2.0 is greater than 1.8
Ms. Shaji has calculated the length of her mansion to be 85,971 Kilometers. Ms. Shaji wants to know how long her mansion is in decimeters. Which is correct? (Unit 1 PAGE 11)
a. 0.85971 dm b. 859,710 dm
c. 859,710,000 dm d. 0.0085971 dm
c. 859,710,000 dm
# 4 WRITE DOWN:
Move the point 4 times to the right
Which of the following phase changes has a decrease in both energy and temperature that changes directly from a gas to a liquid? (Unit 1 PAGE 20 - 22)
a. Freezing b. Evaporation
c. Condensation d. Deposition
c. Condensation
# 9 WRITE DOWN:
Condensation changes Gas to Liquid
Which of the following elements have 3 valence electrons? (Unit 1 PAGE 28 - 29)
a. In, Ga, Al b. Na, Mg, Al
c. Be, Mg, Ca d. Sc, Ti, V
a. In, Ga, Al
# 14 WRITE DOWN:
Group number determines valence electron number
Which element has the highest ionization energy and electronegativity? (Unit 2 PAGE 2-3)
a. Rb b. S
c. C d. Se
b. S
# 19 WRITE DOWN:
S is the most up and right
How does a neutral P atom become a P3- anion? (Unit 2 PAGE 8-9)
a. It gains 3 Protons b. It gains 3 electrons
c. It loses 3 electrons d. It loses 3 Protons
b. It gains 3 electrons
#24 WRITE DOWN:
P becomes more negative with more electrons
Ms. Lyons has a volume of 55,682 milliliters of water. How much water does she have in kiloliters? (Unit 1 PAGE 11)

a. 0.55682 kl b. 55,682,000 kl
c. 5.5682 kl d. 0.055682 kl
d. 0.055682 kl
# 5 WRITE DOWN
Move the point 6 times to the left
What is Letter G? (Unit 1 PAGE 20 - 22)
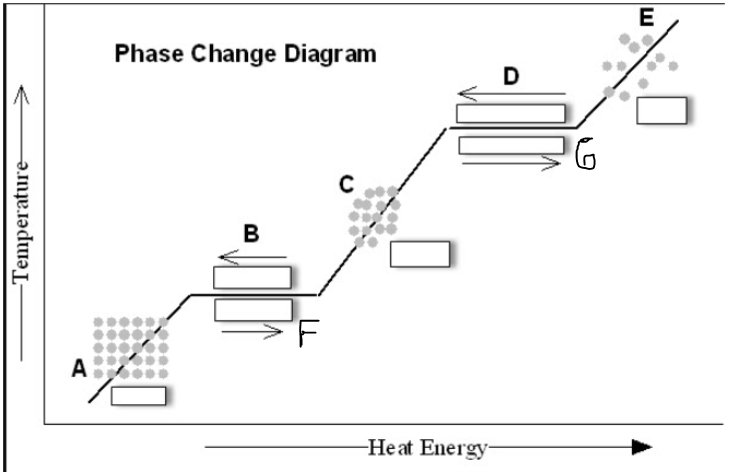
a. Melting b. Condensation
c. Sublimation d. Evaporation
d. Evaporation
#10 WRITE DOWN
Evaporation changes Liquid to Gas
Which of the following elements have the same number of orbitals? (Unit 1 PAGE 28 - 29)

a. He, Ne, Ar b. F, Cl, Br
c. K, Ca, Ti d. N, O, S
c. K, Ca, Ti
# 15 WRITE DOWN:
Period Number determines number of orbitals
Which of the following statements is correct regarding the element Potassium (K)? (Unit 2 PAGE 2-3)

a. It has a smaller atomic radius than Boron.
b. It has more electronegativity than Fluorine.
c. It has a larger atomic radius than Beryllium.
d. It has high Ionization Energy.
c. It has a larger atomic radius than Beryllium.
#20 WRITE DOWN
K is more down and left than Be
Oxygen has gained electrons from Mg. What are the new charges of element O and Mg? (Unit 2 PAGE 8-9)
a. Mg2- and O2+ b. Mg2+ and O2-
c. O1+ and Mg1- d. O1- and Mg1+
b. Mg2+ and O2-
#25 WRITE DOWN:
Mg lost 2 electrons.
O gained 2 electrons.