The number you use to find the number of protons in the nucleus.
What is the atomic number?
3 main states of matter
What are Solid, liquid, and Gas?
Ca
What is the element symbol for Calcium?
B
What is the chemical symbol for Boron?
What is grams?
Three main groups of elements are on the periodic table
What are metals, nonmetals, and metalloids?
Ar
TWhat is the element symbol for Argon?
The number of protons in the atom Beryllium
What is 4?
The chemical formula for Carbon Monoxide.
CO
Avogadro's number
What is 6.02x1023 ?
The trend that means the distance between the nucleus and outermost electron of an atom.
What are atomic radius?
MgCl2
What is the chemical formula for Magnesium Chloride?
Protons and Neutrons
What subatomic particles are in the nucleus?
You must push this button on the scale in order to properly measure using a weight boat.
What is tare or zero?
The proper conversion factor for liters of gas to moles
1/22.4
Group 18 elements on the right hand side of the periodic table of elements.
What are the Noble Gases?
The 7 shown here is called this.
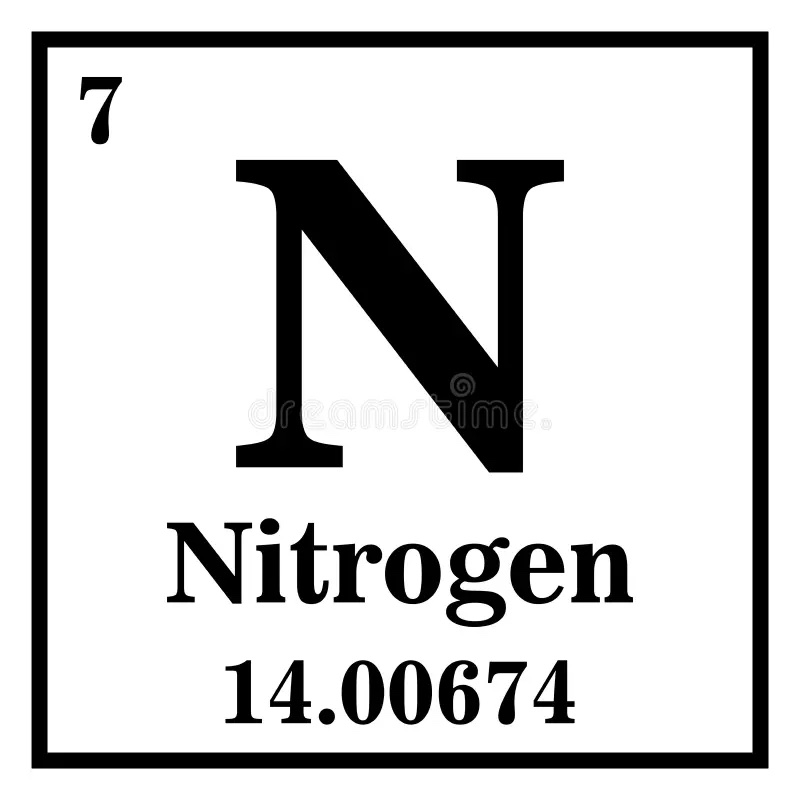
What is the atomic number of Nitrogen?
The electron
What subatomic particle is in the outside of the nucleus of an atom?
Alkali metals
What are the elements in Group 1 called ?
1/(molar mass
What is exchange of electrons?
An ion
What is a positive or negative charged molecule?
Ne
What is the symbol for the element neon?
Halogens
What are the group 17 or 7 elements called?
The volume of 1.25 mol He gas at STP.
What is 28.125 L ?
A covalent bond is formed by
What is the sharing of electrons?
Name all 3 subatomic particles.
What are Protons, Neutrons, and Electrons
Na
What is the element symbol for sodium?
26
How many protons are in Iron?
The mass of 7.00 mol H2O2
What is 238 g ?
The elements in Group 1 or on the left of the periodic table
What are highly reactive elements or alkali metals?
Nitrate, Acetate, Oxalate, Sulfate are all examples.
What is a polyatomic ion?
The type of Elements that are the most abundant on the periodic table.
What is a Metal?
A type of element with properties between metals and non-metals
What are metalloids?
The percent composition of Na3PO4
What is
Na = 42%
P = 19%
O = 39%