Keep it to.....
one page
what is this calculation?
(actual/theoretical) * 100%
percent yield
which of the following has the same trend on the periodic table?
atomic size
electron affinity
ionization energy
ionization energy and electron affinity
what is the ground state electron config of Ar?
1s2 2s2 2p6 3s2 3p6
what is the difference between qsoln and qrxn
the sign
bakers work in grams....
chemist work in moles
what type of structure is not shown?
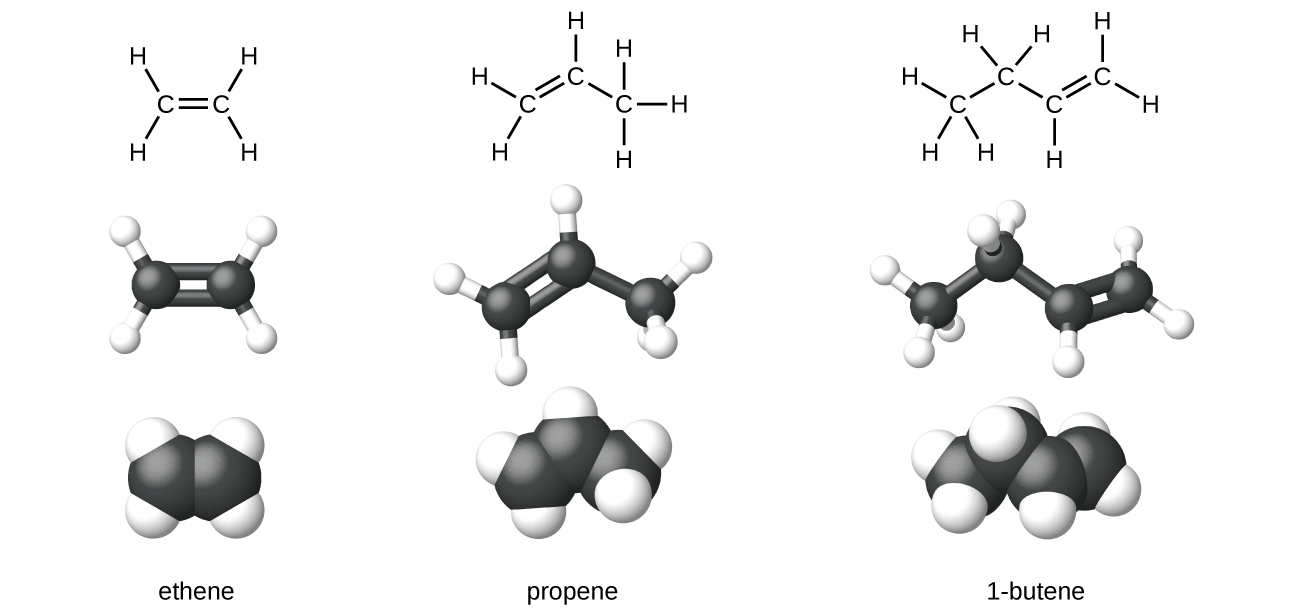
skeleton structure
which element has a larger atomic size?
Li and Ca
Ca
what noble gas would you use to write the short hand electron config of Ti?
Ar
if you have 50.0g of 1M HCl with a density identical to water, how many moles do you have?
0.05 mol
NaOH is commonly used as...
liquid drano
Approximately 80% of organic reactions involve a step called “reflux”. What does it mean to “reflux” an organic mixture?
Reflux is a process in which a solvent is used to keep substances dissolved and at a constant temperature by boiling the solvent, condensing it, and returning it to the flask.
which has the highest IE2 ?
Na, Mg, Al
Na
how many valence electrons does Sn have?
4
if delta T is negative is the reaction endo or exo?
endo
My cat is the...
love of my life
Mass of 2 cups + stir bar=8.27 Mass of 2 cups + stir bar + reactant 1=12.4 Mass of water=100 Mass of 2 cups + stir bar + both reactants=112.4
What is the mass of reaction?
104.13
what is the periodic trend of metallic behavior?
decreases from left to right, and increases down a group
fill in the blanks
periods are...... groups are....
across rows.....down columns
Deterioration of buildings, bridges and other structures as a result of rusting of iron (Fe) costs millions of dollars every day. Although the actual process also requires water, a simplified equation (with rust shown as Fe2O3) is:
4Fe(s) + 3O2(g) → 2Fe2O3(s) ΔHrxn = ‒1.65 x 103 kJ
What is ΔHrxn (in kJ) when 0.250 kg of iron (Fe) rusts?
‒1.85 x 103 kJ
Read the introduction on your own because
that is boring
Hydrogen cyanide (HCN) is produced industrially from the reaction of gaseous ammonia (NH3), oxygen, and methane (CH4). If 5.00 x 106 g each of NH3, O2, and CH4 are reacted, which species is the limiting reactant?
2NH3(g) + 3O2(g) + 2CH4(g) → 2HCN(g) + 6H2O(g)
O2
what is the meaning of isoelectronic?
having the same number of and configuration of electrons as another species
what is the electron config of Cu? (short hand)
[Ar] 3d10 4s1
A student in lab added 4.029 g of solid CaCl2·2 H2O to 49.03 g of deionized water at 20.77 °C in a coffee cup calorimeter. The maximum temperature measured in the calorimeter after the addition of the solid was 26.20 °C. Calculate ΔH (in kJ/mol) for the dissolution of CaCl2·2 H2O. Assume that the solution has the same specific heat capacity as water.
‒44.0 kJ/mol