An element with 16 neutrons, 15 protons and 15 electrons
What is Phosphorus
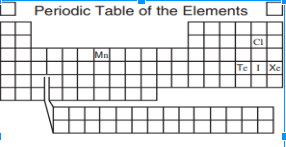 Which of the above elements would have the same properties as Iodine?
Which of the above elements would have the same properties as Iodine?
Manganese , Chlorine, Xenon, Tellurium
What is Chlorine?
The type of radiation that can be blocked by a 2 cm thick cardboard.
What is alpha radiation?
The type of bond where oppositely charged ions attract
What is ionic bond?
The chemical formula for Magnesium Bromate?
What is Mg(BrO3)2?
Where the mass of the atom is mostly concentrated
What is nucleus?
Which of the following elements has both metallic and nonmetallic properties?
Ar ,Ge , Mg , S
What is Ge?
How the atomic mass and atomic number changes when a gamma ray is emitted.
What is no change?
The number of protons and electrons in a
64 29 Cu 2+ ion
What is 29 protons and 27 electrons?
the name of the compound with the chemical formula CrCl3
What is Chromium Chloride?
Which of the following atoms has six valence electrons?
magnesium (Mg)
silicon (Si)
sulfur (S)
argon (Ar)
What is sulfur?
The group in the periodic table with the lowest ionization energy.
What is Alkali metals or group 1?
The decay product represented by X in the decay equation 238 92 U = 4 2 He + X .
What is 234 90 Th ?
The number of bond made on N2, O2, and F2 respectively
What is triple, double and single bond?
the correct name for the acid whose chemical formula is H2SO4
What is Sulfuric Acid?
The element that is represented by this Bohr model
What is Silicon?
The pair of elements that shows an increase in atomic number but a decrease in atomic mass.
Cr and MoPd and Ag
Co and Ni
Ge and Sn
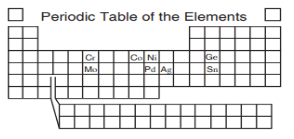
What is Co and Ni?
What is left of an original Polonium sample after three half lives have passed.
What is 12.5%
The charge of Sulfate in Sulfuric acid
What is -2?
The chemical formula for aluminum oxide
What is Al2O3?
Element X has an atomic number of z. Which of the elements below has an atomic number of z + 2 ?
What is G?
Arrange the following elements in order of increasing electronegativity.
F, Be, O, Li
What is Li, Be, O, F?
How many grams of a 10g sample of Osmium with a 21.5 hours half life is left after three half lives?
What is 8.75 g?
The charge of Nitrogen in the compound Nitrogen Triflouride
What is +3?
The name of the compound with a structural formula of
What is Nitrogen Trifluoride?