Open toed shoes are allowed to be worn during labs?
True or False?
What is False
What is the chemical symbol for Gold?
What is
Au
What are the three subatomic particles?
What is
Electrons, Protons, and Neutrons
What is the difference between a core electron and a Valence electron?
What is
A valence electron is on the outermost shell, while core electrons are on the shells inside of the valence shell.
Anions are?
Cations are?
What is
Anions are negative and Cations are positive
Finish the statement:
Matter cannot be created...
What is
nor destroyed
Where is a noble gas located?
What is
Column 8 or Column farthest to the right
How many electrons are in a full valence shell?
What is
8
What group of elements have "full" shells?
What is
The Noble Gases
Why would any atom become an ion?
What is
To "look like" a noble gas and to have a full valence shell
Matter has...
1.
2.
3.
What is
1.mass
2. volume
3. particles
Helium only has 2 electrons, How come it is placed with the noble gases?
What is
Its outermost shell is full.
What correlation is there on the periodic table dealing with atomic masses?
What is
As you go from the left to right atomic masses increase
Mass number - # of Valence electrons = # of _____
What is
# of Core electrons
Does this follow the HONC 1234 Rule? If so, explain why? 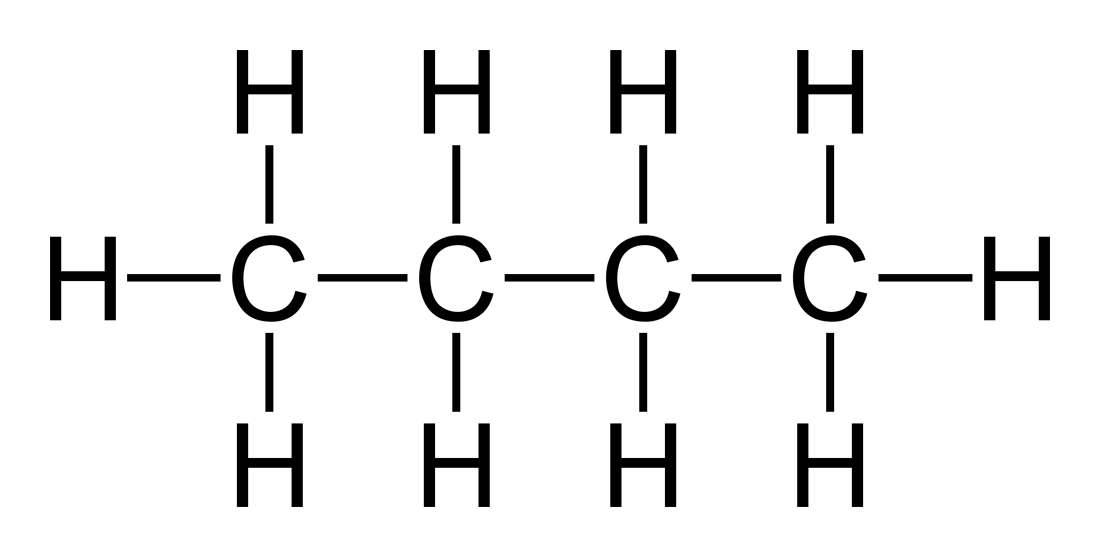
What is
Yes, because all Carbons have 4 bonds, and all Hydrogens have 1 bond
Density = ____ /____
What is
Density = Mass/Volume
Is this a compound?
SCa
What is
Yes
What is the definition of isomers?
What is
atomic of the same elements with a different number of neutrons
Increase in another shell = more _______
Core electrons
You could expect butyl benzoate to smell?
Sweet
Two extensive properties of an object?
What is
Mass and Volume
The periodic table contains metals, ____, and _____
What is
Metals, Non-metals, and metalloids
You have 25% of 37 Cl and 75% of 35Cl what is the average atomic between the two isotopes?
What is
35.5 amu
The farther out you go in a elements valance shells the more _____ there is?
What is
Energy
O=C-O-C
Makes what smell?
A sweet/fruity smell