If the forward reaction of an equilibrium reaction is endothermic, which way does the equilibrium get shifted when temperature is decreased?
Equilibrium shifts to the left.
A Brønsted-Lowry acid is defined as...
a proton donor
The reaction between an acid and a base is called...
a neutralisation reaction
A Brønsted-Lowry base is defined as...
What is reduction
The gain of electrons in a redox reaction.
What is exothermic and draw the corresponding enthalpy graph
High energy to low energy of a substance due to it releasing it to surroundings.
Which of the following has the lowest pH:
HCl, H2SO4, HNO3, HF, CH3COOH, H2CO3, H2O2
H2SO4
What evolves from an acid carbonate reaction?
Carbon Dioxide Gas, CO2 (g)
Why is ammonia considered a weak base?
Because it reacts with water to form ammonium and hydroxide ions, which is at equilibrium (reversible) and only partially dissociates.
What acronym used to memorise electron transfer in redox reactions, and what do the letters represent.
OIL RIG
Oxidation Is Loss Reduction Is Gain
What are the three factors that affect equilibrium?
Pressure, Concentration, Temperature
Acetic acid is a weak acid because...
It only partially dissociates in solution.
In a solution containing 1x10-7 mol L-1 Hydrogen ions and 1x10-7 mol L-1 Hydroxide ions, what is the pH
pH = 7
Is B(OH)3 a strong or a weak base?
Neither, it's a weak acid.

At the cathode
What is a catalyst and how does it affect a reversible reaction
Catalyst provides an alternative pathway, that lowers activation energy. Equally increases the rate of reaction of both forward and reverse reaction
HCl + H2O -> H3O+ + Cl-
What is the Acid and Conjugate Acid
HCl and Cl- is the Acid and Conjugate Acid.
Will the following salt be acidic or basic?
NH3(g) + H2O(l) + HCl(g) -> NH4Cl(aq) + H2O(l)
Acidic: as a weak base reacted with a strong acid
For 0.10 mol L-1 HCl solution at 25 degrees Celsius calculate [OH-]
Kw= [H3O+][OH-]=1x10-14
[OH-]=1x10-14/[H3O+]
[OH-]=1x10-14/0.1
=1.00x10-13
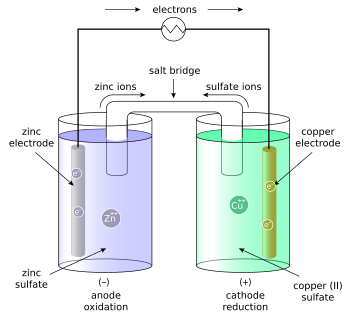
Draw a galvanic cell of Zinc and Copper (and their respective sulfates) labelling: all key features including; electron flow and ion flow.
What is the equilibrium constant of the following equation equal to:
CaCO3(s) + 2H+(aq) <=> Ca2+(aq) + CO2(g) + H2O(l)
K=[Ca2+][CO2]/[H+]2
What does acid taste like?
Sour
Water is an example of a substance that can act as both an acid and a base, what is this called
An Amphoteric Substance
What happens when i pour NaOH(aq) on my hand?
Human soap dispenser. (Slippery)
Draw and label an Alkali Hydrogen fuel cell.

