Which type of formula shows an element symbol for each atom and a line for each bond between atoms? (1) ionic (3) empirical (2) structural (4) molecular
2 structural
What is conserved during all chemical reactions? (1) charge (3) vapor pressure (2) density (4) melting point
1) charge
Which particle has the least mass? (1) a proton (3) a helium atom (2) an electron (4) a hydrogen atom
2) an electron
The joule is a unit of (1) concentration (2) pressure (3) energy (4) volume
(3) energy
Name the 2 major combatants in the Spanish Civil War. (who was the fight between?)
answer: Fascist vs. the Anti-Fascists
Fruit growers in Florida protect oranges when the temperature is near freezing by spraying water on them. It is the freezing of the water that protects the oranges from frost damage. When H2O(ℓ) at 0°C changes to H2O(s) at 0°C, heat energy is released. This energy helps to prevent the temperature inside the orange from dropping below freezing, which could damage the fruit. After harvesting, oranges can be exposed to ethene gas, C2H4, to improve their color.
Write the empirical formula for ethene
CH2
Ammonia, NH3(g), can be used as a substitute for fossil fuels in some internal combustion engines. The reaction between ammonia and oxygen in an engine is represented by the unbalanced equation below.
NH3(g) + O2(g) → N2(g) + H2O(g)+ energy
Balance the equation for the reaction of ammonia and oxygen, using the smallest whole-number coefficients.
4 NH3(g)+ 3 O2(g) → 2 N2(g) + 6 H2O(g)+ energy
Given the balanced equation representing a reaction: 2Na(s) + Cl2(g) → 2NaCl(s) + energy
If 46 grams of Na and 71 grams of Cl2 react completely, what is the total mass of NaCl produced? (1) 58.5 g (2) 163 g (3) 117 g (4) 234 g
(3) 117 g
Given the balanced equation representing a reaction: 2NO + O2 → 2NO2 energy
The mole ratio of NO to NO2 is
(1) 1 to 1 (2) 3 to 2 (3) 2 to 1 (4) 5 to 2
(1) 1 to 1
Which formula is an empirical formula?
(1) CH4 (2) C3H6 (3) C2H6 (4) C4H10
(1) CH4
Which reaction releases the greatest amount of energy per mole of reactant?
(1) decomposition (3) synthesis
(2) single replacement (4) fission
(4) fission
What is the mass of an original 5.60-gram sample of iron-53 that remains unchanged after 25.53 minutes? (1) 0.35 g (2) 1.40 g (3) 0.70 g (4) 2.80 g
(3) 0.70 g
A student measures the mass and volume of a sample of copper at room temperature and 101.3 kPa. The mass is 48.9 grams and the volume is 5.00 cubic centimeters. The student calculates the density of the sample. What is the percent error of the student’s calculated density?
(1) 7.4% (2) 9.2%
(3) 8.4% (4) 10.2%
(2) 9.2%
Parts per million is used to express the
(1) atomic mass of an element
(2) concentration of a solution
(3) volume of a substance
(4) rate of heat transfer
(2) concentration of a solution
Which nuclear emission is negatively charged?
(1) an alpha particle (3) a neutron
(2) a beta particle (4) a positron
(2) a beta particle
Given the equation representing a nuclear reaction: H-1+ X → Li-6 + He-4
The particle represented by X is
(1)4Li-9 (2) 5Be-10 (3) 4Be-9 (4) 6C-10
(3) 4Be-9
Ammonia, NH3(g), can be used as a substitute for fossil fuels in some internal combustion engines. The reaction between ammonia and oxygen in an engine is represented by the unbalanced equation below.
NH3(g) + O2(g) → N2(g) + H2O(g) + energy.
Show a numerical setup for calculating the mass, in grams, of a 4.2-mole sample of O2. Use 32 g/mol as the gram-formula mass of O2.
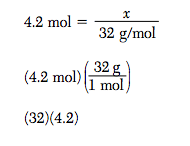
According to Table F, which ions combine with chloride ions to form an insoluble compound?
(1) Fe2+ions (2) Li+ions (2) Ca2+ ions (4) Ag+ions
(4) Ag+ions
Given the balanced equation representing a reaction: 4NH3(g) +? 5O2(g) → 4NO(g) +? 6H2O(g)
What is the number of moles of H2O(g) formed when 2.0 moles of NH3(g) react completely?
(1) 6.0 mol (2) 3.0 mol (3) 2.0 mol (4) 4.0 mol
(2) 3.0 mol
Fission and fusion reactions both release energy. However, only fusion reactions
(1) require elements with large atomic numbers
(2) create radioactive products
(3) use radioactive reactants
(4) combine light nuclei
(4) combine light nuclei
Fruit growers in Florida protect oranges when the temperature is near freezing by spraying water on them. It is the freezing of the water that protects the oranges from frost damage. When H2O(ℓ) at 0°C changes to H2O(s) at 0°C, heat energy is released. This energy helps to prevent the temperature inside the orange from dropping below freezing, which could damage the fruit. After harvesting, oranges can be exposed to ethene gas, C2H4, to improve their color.
Determine the gram-formula mass of ethene.
28 g/mol to 28.1 g/mol