Caused by pollution released into the atmosphere.
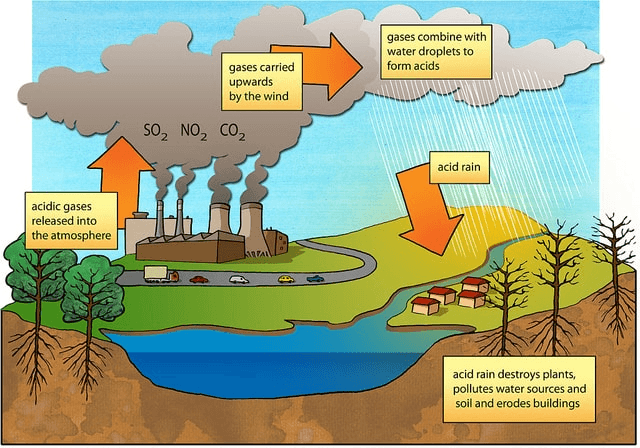
What is acid rain?
Tastes sour.
What is an acid?
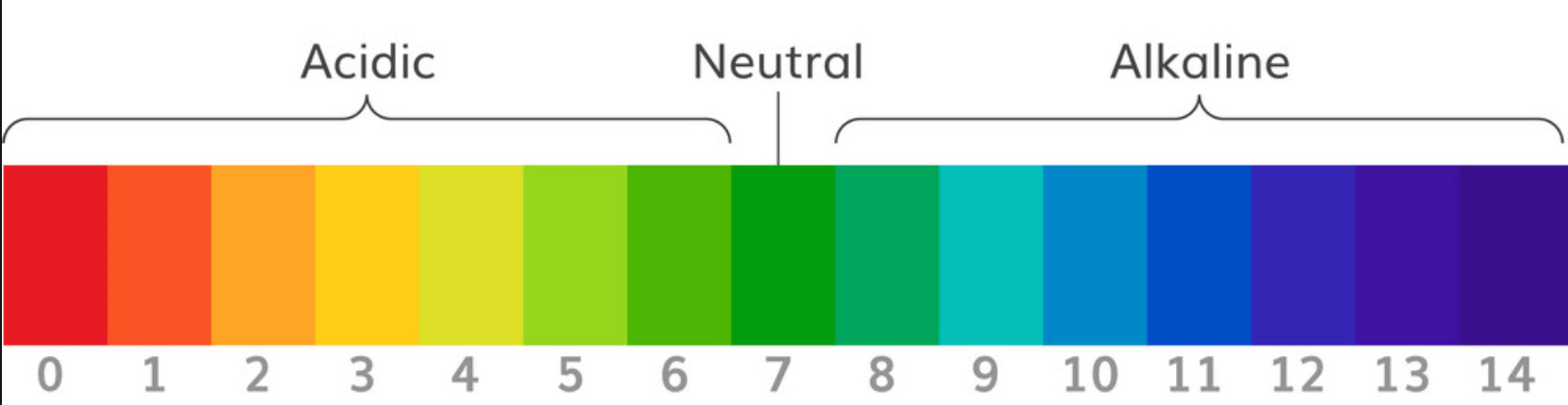
What is the pH scale?
Vinegar
What is an acid?
The pH of water.
What is 7?
The products of a neutralization reaction.
The gas released when a metal reacts with acid.
What is hydrogen (H2)?
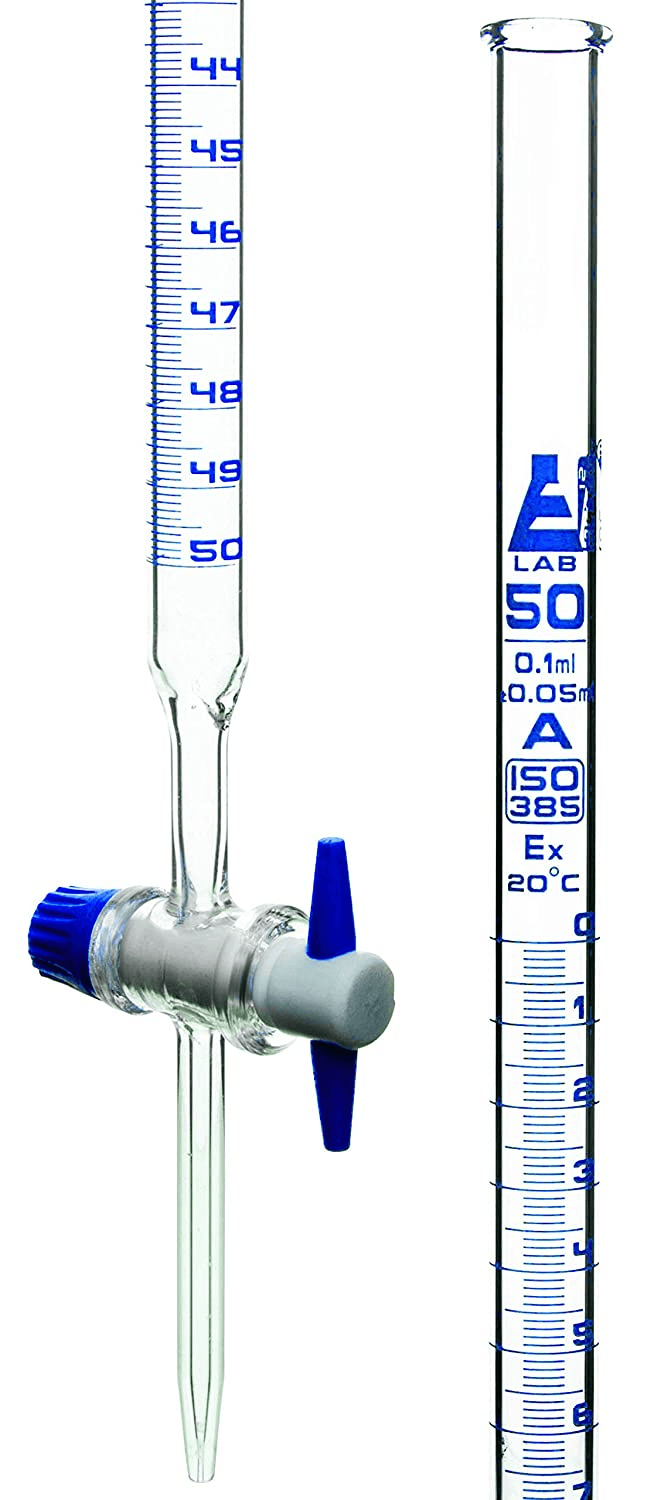
What is a burette?
A consequence of acid rain.
What is killing plant life, erodes limestone or pollutes rivers and lakes?
Tastes bitter.
What is a base?
Numbers in the pH scale that represent acidic solutions.
What is numbers lower than 7?
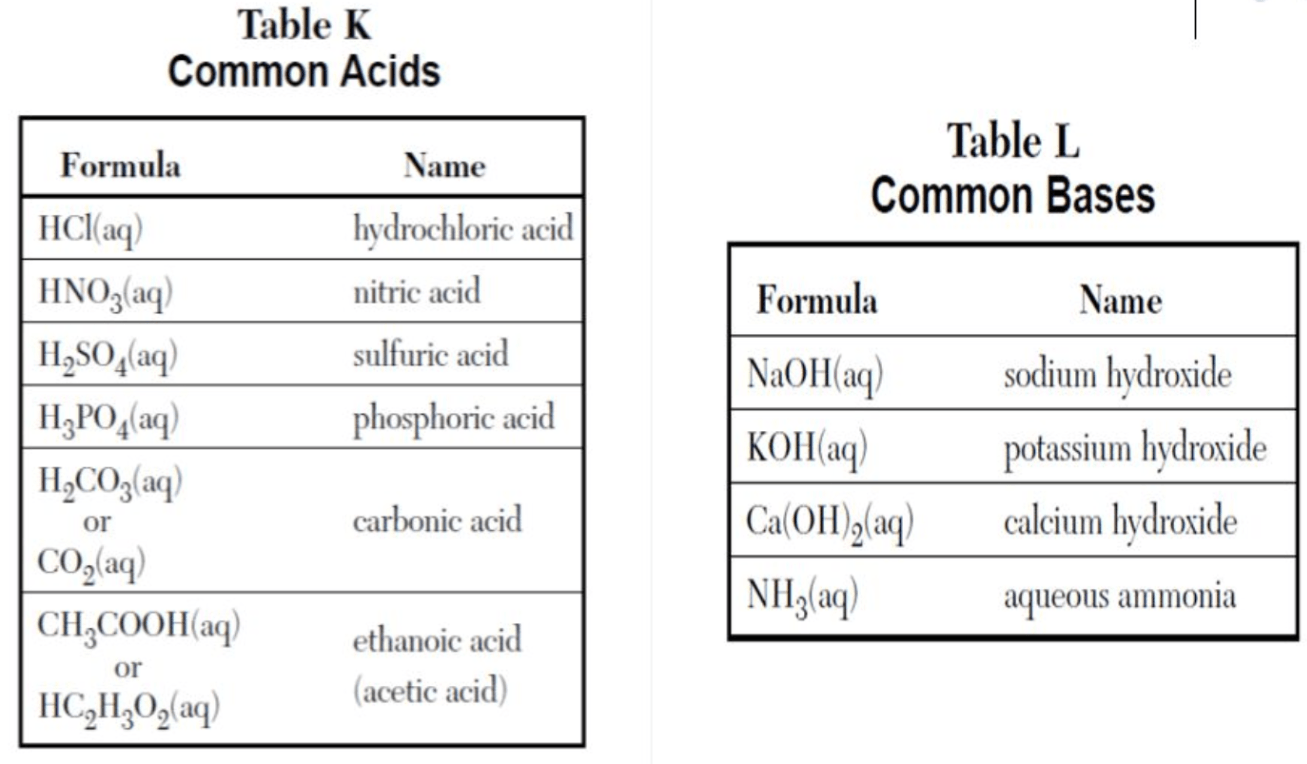
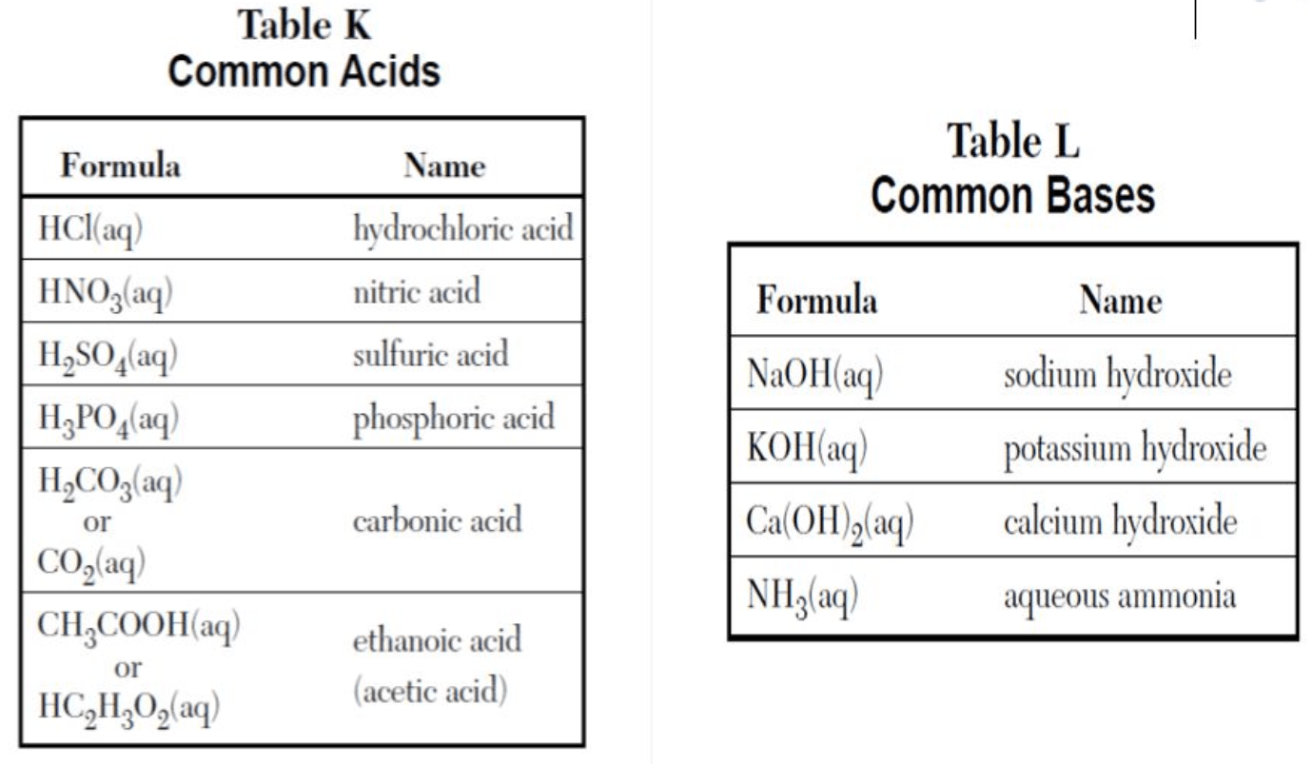
HCl
What is an acid?
(see table K)
Reaction between an acid and a base.
What is a neutralization?
A salt
What is an ionic compound?
A reference table that would help you decide which metals will react with an acid and which ones will not.
What is table J?
The lab procedure used to find the unknown concentration of a solution by reacting it with another solution of known concentration.
What is a titration?
The product of combining H+ ions with H2O.
What is a hydronium ion (H3O+)?
Reacts with some metals to produce hydrogen gas.
What is an acid?
Numbers in the pH scale that represent basic (alkaline) solutions.
What is a number higher than 7?

Usually referred to as alkaline.
What is a base?
The pH number that represents a neutral solution.
What is pH = 7?
The salt that results from the neutralization of HCl with KOH.
What is KCl?
(The H from the acid and the OH from the base make H2O. Whatever is left forms the salt.)
A metal that will NOT react with an acid.
What is copper (Cu), silver (Ag) or gold (Au)?
(any metal below H2 in table J)

The mathematical equation used to make calculations using data from a titration.
What is
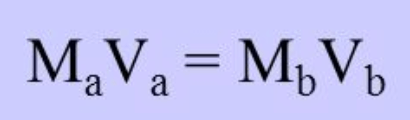
Area in the United States where the most acidic rain occurs.
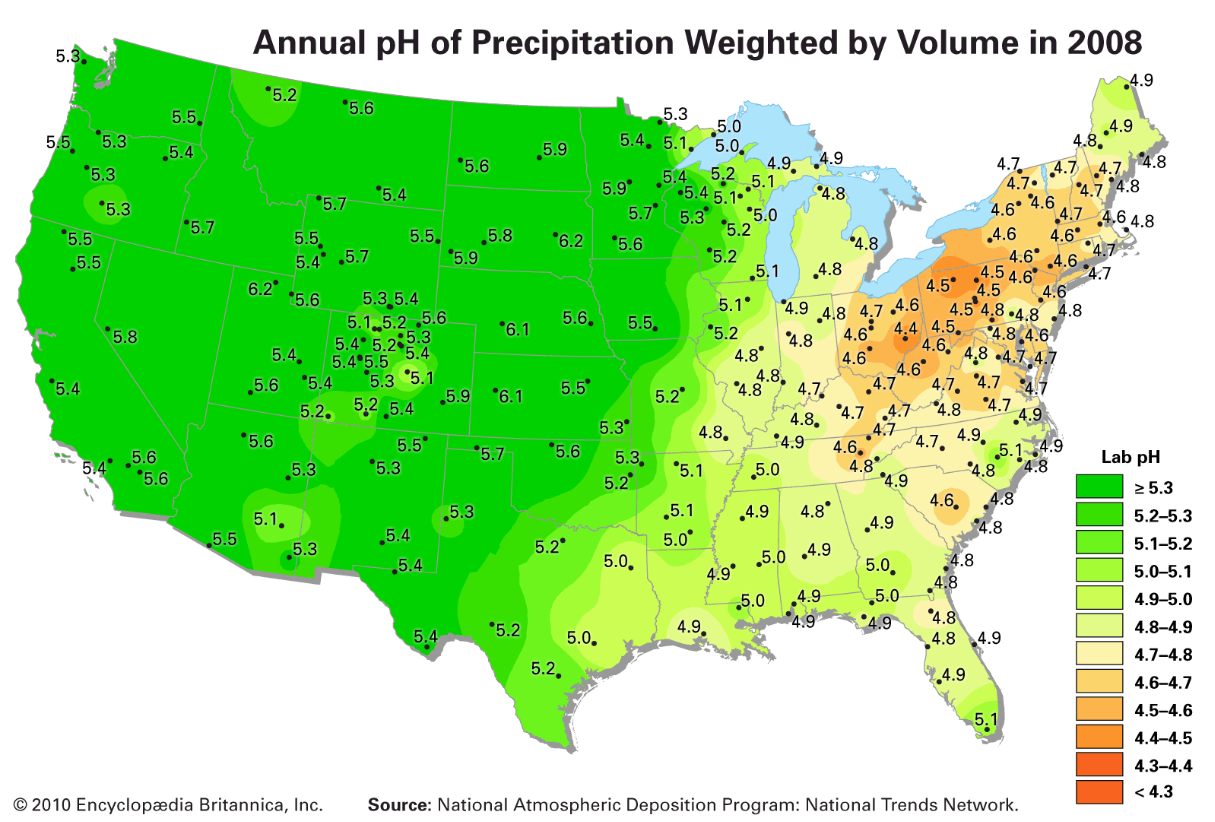
What is the north east area?
(yellow/orange colors represent lower pH = more acidic)
Produces OH- (hydroxide ions) when dissolved in water.
What is a base?
Compares the acidity of lemon juice (pH 2) and stomach acid (pH 1)
What is stomach acid is 10 times MORE ACIDIC than a lemon juice? or lemon juice is 10 times LESS ACIDIC than stomach acid?
H2SO4
What is an acid?

The product of combining H+ and OH-.
What is water (H2O)?
The salt that results from the neutralization of HNO3 and NaOH.
What is NaNO3?
(The H from the acid and the OH from the base make H2O. Whatever is left forms the salt.)
The metal shown here reacting with an acid.
Mg + 2HCl -> MgCl2 + H2
What is magnesium (Mg)?
Must be added to the solution being titrated in order to identify the endpoint of the titration.
What is an indicator?
The gas produced in the reaction between an acid and a carbonate (like limestone). See reaction.
CaCO3 + H+ -> Ca+2 + H2O + CO2
What is carbon dioxide (CO2)?
Produces H+ (hydrogen ions) when dissolved in water?
What is an acid?
Compares the acidity of a solution with a pH of 6 and a solution with a pH of 4.
What is a pH of 6 is 100 times LESS ACIDIC than a pH of 4?
Baking soda
What is a base?
If acid spilled on a river, this would happen to the pH of the river water.
What is a decrease in pH?
(recall that acids have low pH values)
H2SO4 + Ca(OH)2 -> 2H2O + _______
What is CaSO4?
(The H from the acid and the OH from the base make H2O. Whatever is left forms the salt.)
A metal that WILL react with an acid to produce hydrogen gas.
What is Pb, Zn, Al, Na...?
(mention any metal that is above H2 on table J)

The solution color that will signal the correct time to stop the titration.

What is light pink? or the flask at the center?
Explains why the reaction between acid rain and limestone is very slow.
What is the acid concentration in rain is low (compared to, let's say, vinegar), and rain water is not always in contact with the limestone?
Conducts electricity when dissolved in water.
What is an acid or a base?
(Sorry, trick question. Both acids and bases are conductors of electricity :)
The pH of a solution 1000 times MORE ACIDIC than water.
What is a pH of 4?
(recall that water's pH =7)
KOH
What is a base?

The reason why antiacid tablets, like Tums, help with acid reflux.
What is acid from the stomach that is causing the acid reflux gets neutralized by the antiacid (alkaline) tablet, minimizing the destructive effect of the acid in your esophagus?
The acid and base that formed KNO3 in a neutralization reaction.
What is HNO3 (acid) and KOH (base)?
A possible reason that explains the difference in reaction rate between magnesium and hydrochloric acid shown here.

What is a difference in concentration, the first tube having the most concentrated acid and the last tube having the lowest concentration?
(High concentration, faster rate of reaction)
The molarity of a 20.0 mL sample of an acid that was titrated with 10.4 mL a 0.30M KOH solution.
What is 0.156 M?
MA (20.0mL) = (0.30M)(10.4mL)