The following are characteristics, but only some of them are considered characteristics of reliable science:
Biased, Replicable by other scientists, Repeated testing, Controlled variable, Paid sponsorship
What is controlled variable, repeated testing and replicable
The amount of moles of sodium (Na) in a sample of 5.87 × 1024 atoms of sodium?
What is 9.75 moles Na
At what "point" in a cooling curve are both a liquid and solid present?
What is the freezing point
Temperature is the measure of _________ ?
This property of water causes it to attract to other water molecules.
What is polarity (positive/negative ends to water)
The amount of mL that is equal to 42 L.
What is 42,000 mL
The amount of molecules in 252.3 g H2S?
What is 4.458 × 1024 molecules H2S
The theory that contains postulates such as gas particles are in constant motion, and there is no attraction or repulsion between gases is referred to as?
What is the Kinetic Molecular Theory
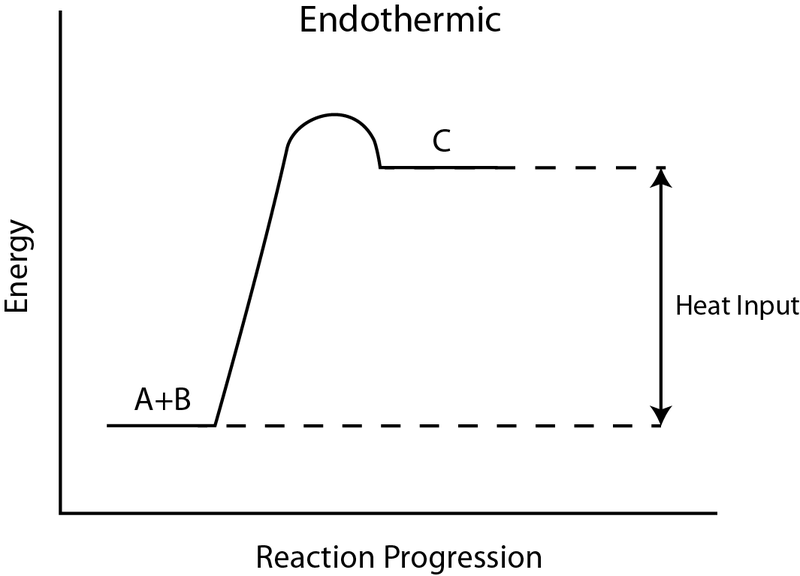
The "heat input" on this graph represents what change?
What is change in enthalpy
If you had a jug full of lemonade and added more water to it, what would happen to the Molarity of the solution?
It would decrease (adding more volume of water)
The electron configuration for arsenic (As).
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
The empirical formula of a compound that is 75.74 percent arsenic and 24.26 percent oxygen.
What is As2O3
When 2.00 L at 21.0 °C is compressed to 1.00 L, what is the resulting temperature (in Celsius)?
−126.0 °C (must convert temp to kelvin and convert back)

This reaction would have an overall energy change of?
-35 kJ (exothermic)
How many moles of NaCl would be needed to produce a 0.5 M solution that is 500 mL total?
0.25 moles of NaCl (make sure to convert mL to L)
 This "plum pudding" model was created by what scientist?
This "plum pudding" model was created by what scientist?
JJ Thomson
When 4.0 mol H2 react with 8.0 mol O2 in the reaction 2H2 + O2 → 2H2O, what are the theoretical yield (in moles) and the limiting reactant?
H2 limiting, theoretical yield is 4 mol H2O
If an excess of hydrogen gas reacts with 22.0 g nitrogen, how many liters of ammonia can be produced at STP? The balanced equation is 3H2 + N2 → 2NH3.
35.2 L NH3
The activation energy of this graph is?
200 kJ
How many grams of NaOH (molar mass = 40 g/mol) would you need to prepare 1 L of 1.5 M solution.
60 grams
Draw the Lewis structure for CCl2O. The correct name of this molecular geometry of this structure is?
What is trigonal planar
If 80.0 grams of I2O5 react with 28.0 grams of CO, what mass of I2 can could be produced?
Balanced equation: I2O5 + 5CO → 5CO2 + I2
50.8 g I2 theoretical yield
At what temperature will 0.654 moles of argon gas occupy 12.30 liters at 1.95 atmospheres?
447 K
2SO2(g) + O2(g) ⟶ 2SO3(g), △H= - 198 kJ
What is the enthalpy change that occurs when 4 moles of SO2 reacts with excess oxygen in the above reaction?
-396 kJ (double the amount)
If you have 14 M solution of Hydrochloric Acid, HCl, and want to dilute it to make 2 L of a 2 M solution, what volume of the original solution would you need?
0.29 L