Matter that contains only one kind of particle.
Pure Substance
The larger part of a solution, the part into which the solutes dissolve
Solvent
What is Sorting?
Physically separating large pieces of a mechanical mixture so that similar pieces are together
When looking at a picture how do a mechanical mixture and a solution differ?
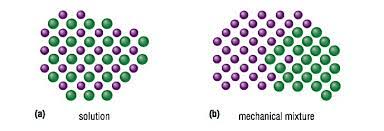
When there is not very much of a solute dissolved in a solution it is called?
Dilute
Matter that contains two or more pure substances mixed together
Mixture
The smaller part of a solution, the part of a solution that dissolves the solvent
Solute
What is Floating?
A separation technique in which the ligher component rises to the top of a liquid
Give an example from class of a solute that did dissolve well in water.
Salt & or Sugar.
Which method would you use to remove oil from a mechanical mixture. How does this connect to an oil spill.
Floating.
When cleaning up an oil spill, they use floating booms and skimmers to collect the oil on top of the ocean.
A mixture with different parts that you can see
Mechanical Mixture
The amount of solute present in an amount of solution
Concentration
What is settling?
A separation technique in which the heavier component sinks to the bottom of a liquid
Give an example of a solute that does not dissolve in water?
Cooking Oil.
What is the most common solvent on earth?
Water
A mixture that looks like a single pure substance; a uniform mixture of two or more pure substances
Able to dissolve in a specified solvent
Soluable
What is a sieve?
A device used to separate the components of a mixture, with many visible holes that allow smaller solids and liquids to pass through
When no more epsom salts would dissolve into our solution - it is called....
A saturated solution
What method would you use to separate salt from water?
To mix one type of matter into another type of matter to form a solution
Dissolve
Not able to dissolve in a specified solvent
Insoluable
What is a Filter?
A device with many small holes that trap solid pieces of a mixture but allow liquids and gases to pass through
What is the formula for the concentration of a solution?
Concentration formula = mass of solute/100 ml of a solution
Connect your separating mechanical mixtures lab to how hard it is to clean up an oil spill.
When oil is spilt it is very very difficult to completely clean everything and remove oil from the wildlife... kind of like how we couldn't clean all the seeds etc. during our experiment.