The name for Ca3P2.
What is calcium phosphide?
A type of bond characterized by the sharing of electrons by the overlapping of atomic orbitals, usually between nonmetals.
What is a covalent bond?
The shape and polarity of CH2Br2.
What is tetrahedral and polar?
The balanced equation and category of the reaction:
KClO3 ⟶ KCl + O2
What is decomposition and
2 KClO3 ⟶ 2 KCl + 3 O2 ?
The molar mass of trisodium phosphate.
What is 163.94 g/mol?
The type of solutions that have pHs from 0 - 6.bar9 are sour.
What are acids?
The chemical formula for tetrafluoride pentabromide.
What is F4Br5?
The reason that group 18 elements rarely form bonds.
What is a full valence shell? (ns2p6, 8 electrons)
The shape and polarity of an ammonia molecule.
What is trigonal pyramid and polar?
The completed balanced equation and category of the reaction
Ca(NO3)2 + Na ⟶ .
What is single displacement and
Ca(NO3)2 + 2 Na ⟶ 2 NaNO3 + Ca ?
The number of atoms in two moles of sodium atoms.
"2 mol Na"*(6.022\times10^23 "atoms")/"1 mol" = 1.204\times10^24 "atoms Na"
The type of solutions that have an equal concentration of hydronium and hydroxide.
What is neutral?
The name of the compound Co2O3.
What is cobalt (III) oxide?
The classification of a covalently bonded molecule that has a moderate difference in electronegativity across the molecule.
What is polar?
The shape and polarity of a CO2 molecule.
What is linear and nonpolar?
The balanced equation and category of the reaction:
Al + O2 ⟶ Al2O3
What is synthesis and
4 Al + 3 O2 ⟶ 2 Al2O3 ?
The moles of atoms in 8.43 \times 10^24 molecules of CH4.
8.43\times10^23"molecules CH"_4*(1"mol CH"_4)/(6.022\times10^23"molecules")*"5 mol atoms"/"1 mol CH"_4 = "70.0 mol atoms"
The pH of a solution with [H^+]=1.602\times10^-4.
What is 3.795?
The chemical formula for manganese(IV) nitrate.
What is Mn(NO3)4?
The ionic compounds of the series Na3PO4, Co(OH)2, H3PO4, AsCl3, AlCl3, COOH2.
What are Na3PO4, Co(OH)2, and AlCl3?
The Lewis structure for sulfur tetrafluoride.
What is _?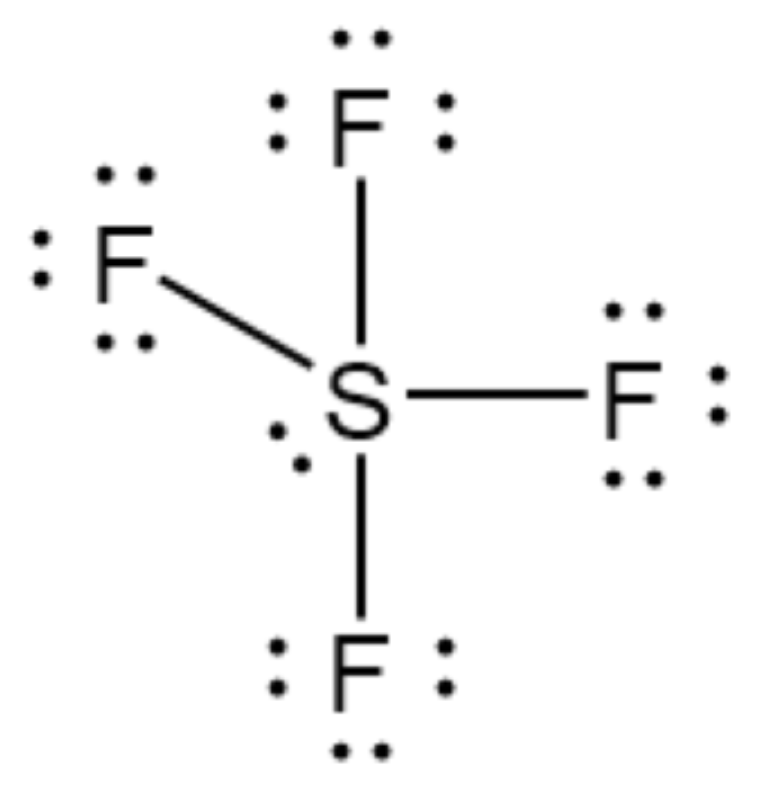
The type of reaction and the balanced chemical equation representing "manganese (V) chloride reacts with fluorine gas".
What is single displacement and
2 MnCl5 + 5 F2 ⟶ 2 MnF5 + 5 Cl2 ?
The number of atoms in 64.32 g of zinc.
"64.32 g Zn"*"1 mol Zn"/"65.38 g Zn"*("6.022"\times10^23" atoms")/"1 mol Zn" = 5.92\times10^23" atoms"
The [H+] of a solution with a pH of 9.23.
What is 5.89 \times 10^-10 ?
The name of B6Si.
What is hexaboron monosilicide?
A category of elements characterized by the ability to form various oxidation states due to the strange mechanics of d orbitals.
What are transition metals?
The Lewis structure for the compound C2H2(OH)2.
What is _?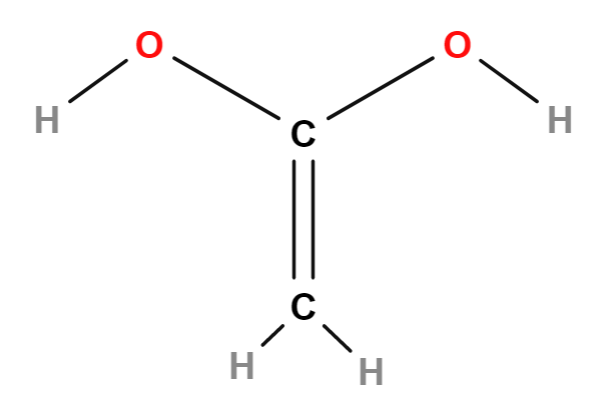
The completed balanced equation and category of the reaction
(NH4)2S + Sr3(PO3)2 ⟶
What is double displacement and
3 (NH4)2S + Sr3(PO3)2 ⟶ 3 SrS + 2 (NH4)3PO3 ?
Cu + Cl2 ⟶ CuCl2
If 12.5 g of Cu reacts with excess chlorine, calculate the theoretical yield of CuCl2.
"12.5 g Cu"*"1 mol Cu"/"63.546 g Cu"*"1 mol CuCl"_2/ "1 mol Cu"*"134.45 g CuCl"_2/"1 mol CuCl"_2 = "26.4 g CuCl"_2
The [OH-] of a 12.7 pH solution.
[H^+] = 10^"-pH"=10^-12.7=2.00\times10^-10 M
[OH^-] = 10^-14/[H^+] = 10^-14/(2.00\times10^-10) = 5.01\times10^-2 M
The chemical formula for ammonium hydrogen sulfate.
What is NH4HSO4?
Draw all resonance structures of ClO3-.
Three structures, each with a different O having the single bond and negative charge.
The shape and polarity of a sulfate molecule.
What is tetrahedral and nonpolar?
The balanced chemical equation representing the combustion of C5H8O3.
What is
2 C5H8O3 + 11 O2 ⟶ 10 CO2 + 8 H2O ?
The mass of solid made from the reaction of 198.7 g of lead(II) nitrate and 232.4 g of potassium iodide.
Pb(NO_3)_"2 (aq)" + "2 KI" \rightarrow "2 KNO"_"3 (aq)"+PbI_"2 (s)"
"198.7 g Pb(NO"_3")"_2*("1 mol Pb(NO"_3")"_2)/("331.2 g Pb(NO"_3")"_2)*("1 mol PbI"_2)/("1 mol Pb(NO"_3")"_2)*("461.01 g PbI"_2)/("1 mol PbI"_2) = "276.6 g PbI"_2
"232.4 g KI"*"1 mol KI"/"166.003 g KI"*"1 mol PbI"_2/"2 mol KI"*("461.01 g PbI"_2)/("1 mol PbI"_2) = "322.7 g PbI"_2
What is 276.6 g PbI2?
The pH of a solution made from adding 43.7 g of Al(OH)3 to 435 mL of water.
"43.7 g Al(OH)"_3 * "1 mol Al(OH)"_3/"78.003 g Al(OH)"_3*"3 mol OH"^-/"1 mol Al(OH)"_3*1/"435 mL"*"1000 mL"/"1 L" = 3.86 M" OH"^-
[H^+] = 10^-14/[OH^-] = 10^-14/3.86=2.59\times10^-15 M
pH = -log[H^+] = -log(2.58\times10^-15) = 14.6