I am a metal, that is in group 1. I am slightly less reactive than Sodium, and more reactive than Beryllium. Who am I?
Lithium

I have 5 Valence Electrons!
Chlorine
Predict what the Lewis Dot diagram for Calcium would look like.
Predict what kind of bond Sodium would most likely make. (Ionic or covalent)
metals form either ionic bonds (metal + non-metal) or metalic bonds (metal + metal)
I am a halogen which lives in group 17. I am working on finishing my second valence shell. Who am I?
Florine
![]()
I have 4 Valence Electrons
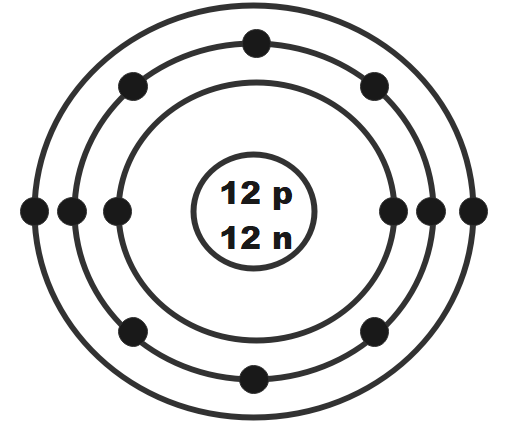
Magnesium
Predict what the Lewis Dot diagram for Oxygen would look like.
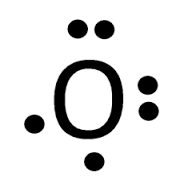
Predict the first ever element ever formed in the universe.
Hydrogen
I am a metal that has a golden lustre and am commonly used in computer chip manufacturing and jewellery. Who am I?
Gold

I have 2 Valence Electrons
Carbon
Predict what the lewis dot diagram for Neon would look like.

Predict the number of protons the element with an atomic number of 10 would have.
10
I already have a full valence shell, I live in period 3, who am I?
Argon
I have 7 Valence Electrons
![]()
Silicone
Predict what the Lewis Dot diagram for Hydrogen would look like.
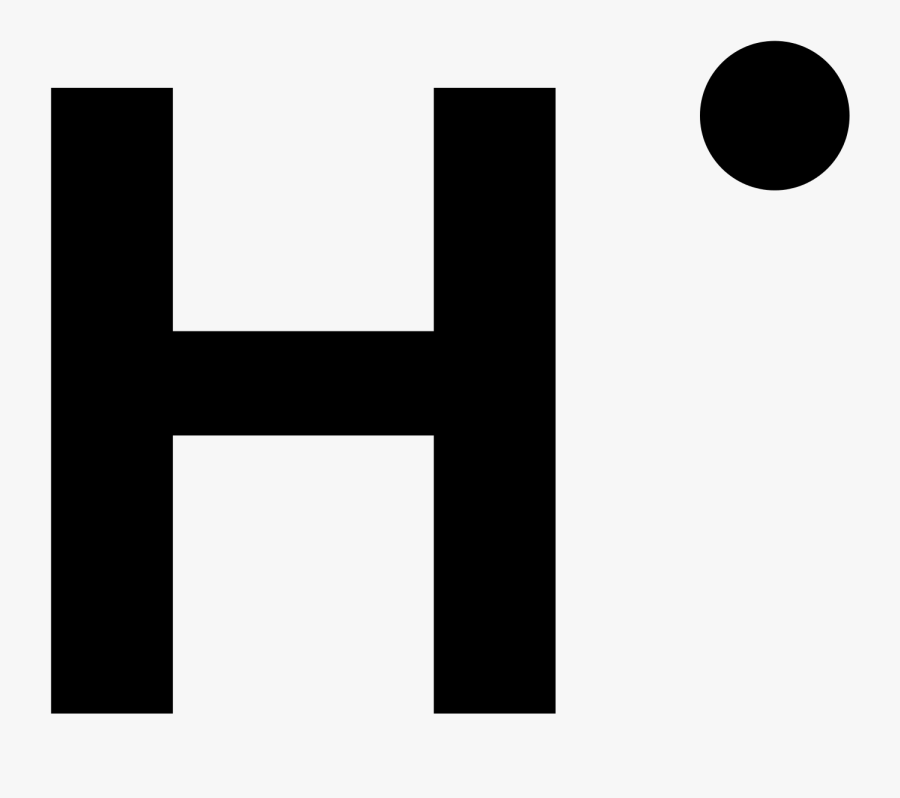
What does the number of valence electrons indicate.
The number of electrons that exist in the outermost shell.
We are almost noble, and together we form one of the most common seasonings. Who are we.
Sodium and Chlorine.

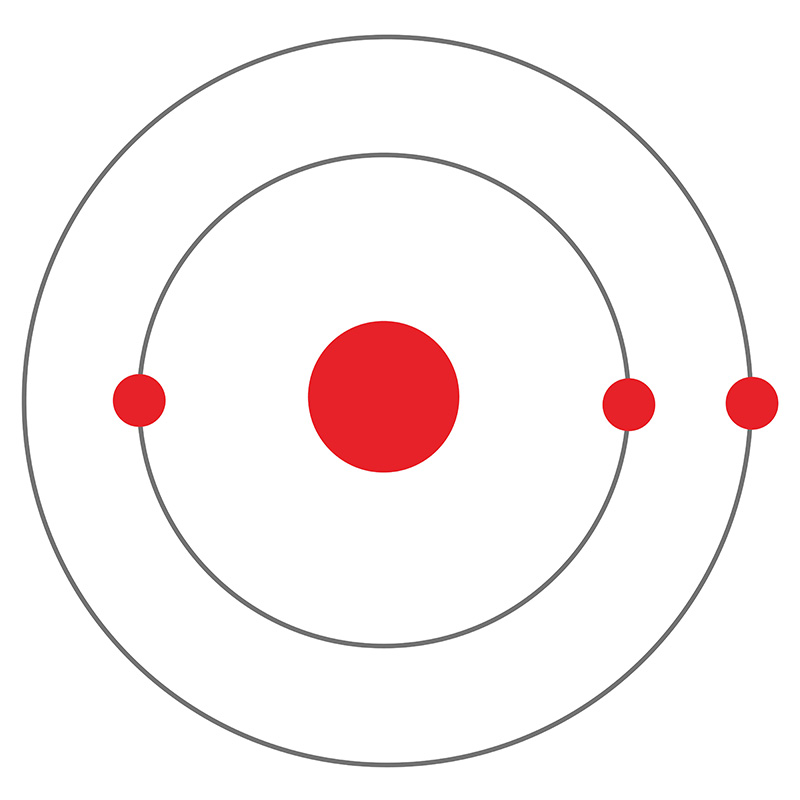
Lithium
Predict what the Lewis Dot diagram for Sulphur would look like
Double Point Question: Which group are alkaline metals most likely to react with?
Halogens