What is Energy?
Ability to do work or produce heat
What is the law of conservation of matter?
Matter is never created nor destroyed.
What is a proton and what is it charge?
positively charged subatomic particle.
Whose model of the atom is this?
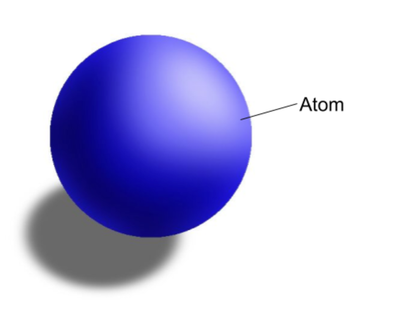
John Dalton
Define Element:
Substance that cannot be separated into simpler substances by a chemical change.
What is kinetic energy and what is a good example?
Teacher approves the answer
What does heterogeneous mean?
Two or more, different
What is a neutron and what is its charge
Whose model of the atom is this?
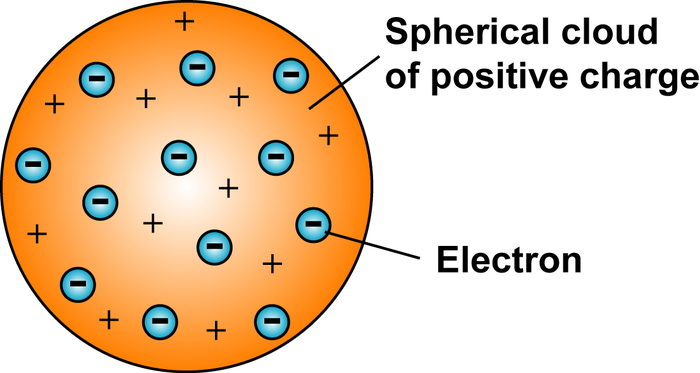
J.J. Thompson's model
Define Compound:
Two or more elements chemically combined.
What is potential energy and what is an example?
Teacher approval
What does homogeneous mean?
Same
What is an electron and what is its charge?
Negatively charged subatomic particle
Whose model of the atom is this?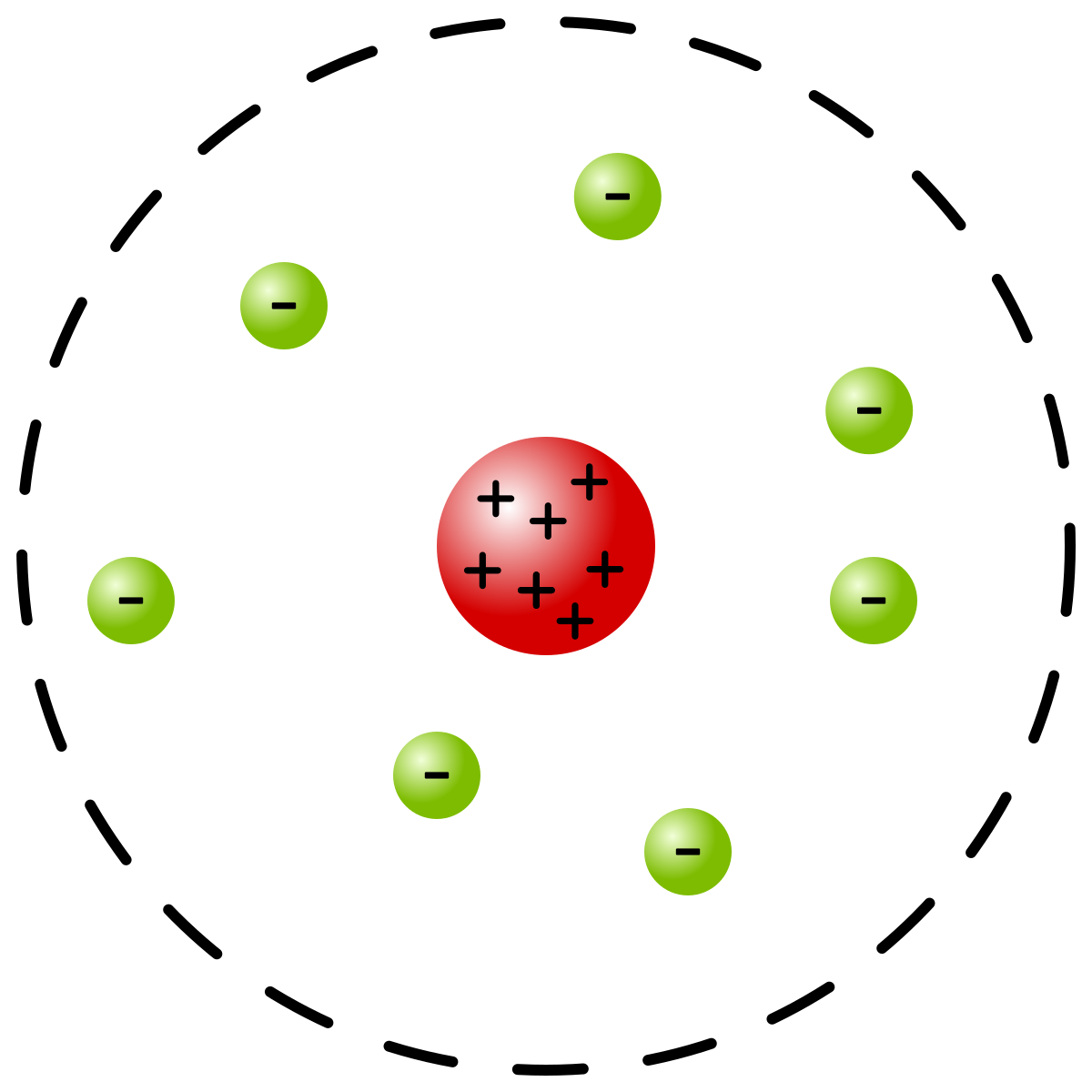
Earnest Rutherford's model
Define Mixture:
A blend of two or more pure substances
Matter is define as anything that has ______ and ______?
Mass and volume
What does subatomic mean?
Smaller than an atom
Which two subatomic particles are located in the nucleus of an atom?
Nucleus and Proton
Whose model of the atom is this?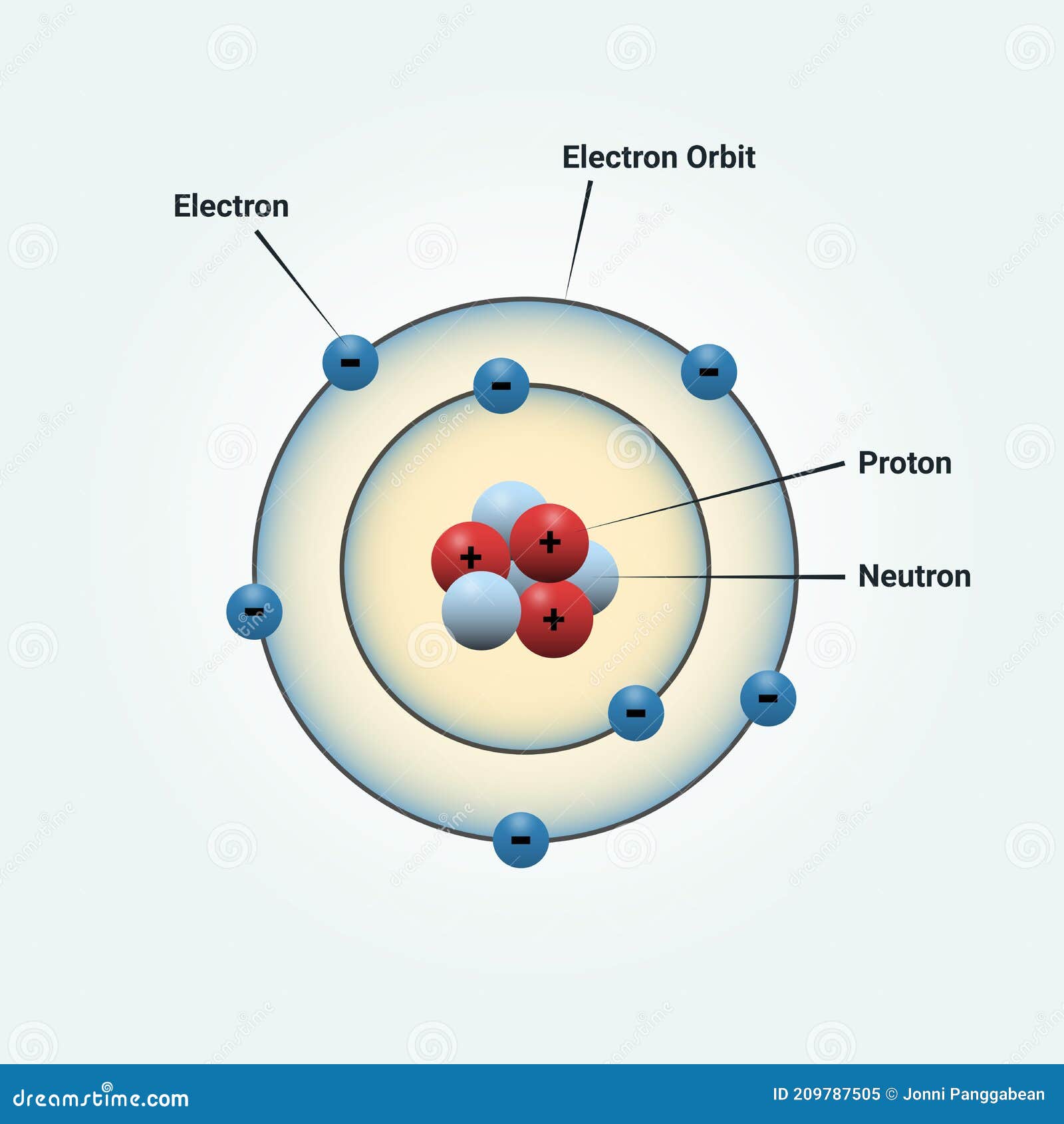
Niehl's bohr
Teacher approval
What is an atom?
Smallest particle of an element that retains the chemical identity of the element.
What is a pure substance?
Substances that are made up of only one kind of particle.
Look to Mr. B for your question
Teacher approval
What is atomic theory?
Theory of what humankind thinks atoms look like.
Given an example of homogeneous mixture
Teacher approval