Which particle in an atom has a negative charge?
Electron
The periodic table is arranged by atomic _________.
Number
Chemical or physical property?
Density
What are the reactants of this equation?
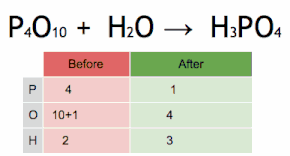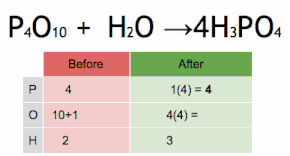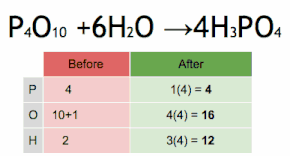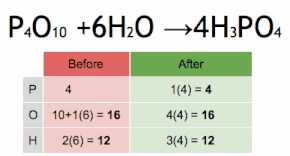
P4O10 and H2O
What is the definition of the Law of Conservation of mass?
Mass/matter cannot be created or destroyed.
What is in the nucleus of an atom?
Proton and neutron
What is another name for columns on the periodic table?
Groups/Families
Chemical or physical property?
reacts with water
Chemical
How many Oxygen atoms are in the reactants of this equation?

18
What is an open system?
What does the atomic number tell you about an atom?
The number of protons (which will be the same as the electrons)
Periods
Chemical or physical change?
Dissolving sugar in water
Physical
How many Oxygen atoms are in the product of this reaction?

6
20g of a substance reacts with 12g of another substance. What will be the mass of the product?
What is a valence electron?
An electron on the outer shell
What is the name of group 17 (7A) on the periodic table?
Halogens
Chemical or physical change?
baking pie
Chemical
C6H12O6 + 6O2→6CO2 + 6H2O
Is this equation balanced?
No
10g of a substance reactants with another substance. The products are 15g of one substance and 30g of another. What is the mass of the other reactant?
35g
What does the atomic mass tell you?
The mass of the protons and neutrons in the nucleus.
Were will elements with similar properties be on the periodic table in relation to one another?
in the same group
What type of mixture is chex mix?
Heterogeneous
2 Fe + 3 Cl2 = 2 FeCl3
Is this equation balanced?
Yes
A reaction occurs with 10g of a substance and 20g of another. The mass of the product is 25g. What happened to the other 5g?
Given off as gas.