What is Heisenberg's uncertainty principal?
We cannot know both the position and speed of a particle
What is an orbital?
Areas with a high probability of finding e-
How many sublevels are in quantum number 4?
4
Show the correct e- config of iron (Fe)
T or F The rows on the periodic table are called periods
T
What was established about triads?
The middle element is the average of the outer two elements
Describe Aufbau principle (where are electrons added first)
E- are added one at a time to the lowest energy orbital
Which correctly shows the total number of e- and shape of S orbitals?
Sphere, 2e- total
Nitrogen
T or F The vertical columns on the periodic table are called periods
F
How were early periodic tables first organized
Atomic mass/ properties
Describes Pauli principle (how many can each orbital hold of opposite spin)
Orbitals can hold 2 E- with opposite spin
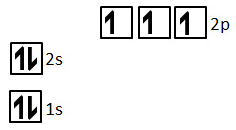
Which correctly shows the total number of e- and shape of P orbitals?
Dumbbell, 6e- total
Give electron configuration of the following: Lu
[Xe]6s2 4f14 5d1
Give electron configuration of the following: In
[Kr]5s2 4d10 5p1
Why did Mendeleev organize his periodic table somewhat by properties rather than mass?
He believed the mass measurements were wrong
Correctly describes Hund's rule (Do E- spread out or pair first?)
E- spread out within the orbital before pairing
Which correctly shows the total number of e- and shape of d orbitals?
Flower shape, 10 e- total
Give electron configuration of the following: B
[He]2s2 2p1
Why was J.A.R Newland made fun of?
He introduced the idea of octaves which related science to music
Who discovered atomic #?
HGJ Mosley
How many elements are in the 6th period of the periodic table
32
Which correctly shows the total number of e- and shape of f orbitals?
7 types of unique shapes, 14 e- total
Give electron configuration of the following: P
[Ne]3s2 3p3
What is the name of family 1A
What is the name of family 2A
What is the name of family 7A
What is the name of family 8A
Alkali metals
Alkaline earth metals
Halogens
Noble gases