What unit for volume is used for gases?
L (Liters)
What does a manometer measure?
What do the variables P and V stand for in Boyle's Law?
P = pressure
V = volume
Convert 965 mmHg to atm
1 atm = 760 mmHg
1.27 atm
When one variable is increased, it causes the other variable to increase as well.
Are these variables directly or indirectly related?
directly
Give three units for pressure.
mmHg
atm
Pascals
Kilopascals
torr
psi
Name the two types of manometers.
Open-end manometers, close-end manometers
What law relates temperature of a gas to its volume?
Charles' Law
Convert 77.0 L at 18.0 mmHg to its new volume at standard pressure.
Standard pressure = 760 mmHg
3251 L
Gay-Lussac's law of pressure and temperature has the formula
P1/T1 = P2/T2
Are pressure and temperature directly or indirectly related?
indirectly
What unit of temperature must be used in any gas law calculation?
Kelvin

Which is greater, the pressure of the gas or the pressure of the atmosphere?
the pressure of the atmosphere
Write the formula for the Ideal Gas Law.
PV = nRT
How many moles of CO2 are needed to fill an 80.0 L tank to a pressure of 150.0 atm at 300.15 K?
PV = nRT
R = 0.0821
0.0021 mols CO2
What would happen to the pressure of a gas if temperature was increased and volume was decreased?
increase
How many mmHg are in one atm?
760 mmHg
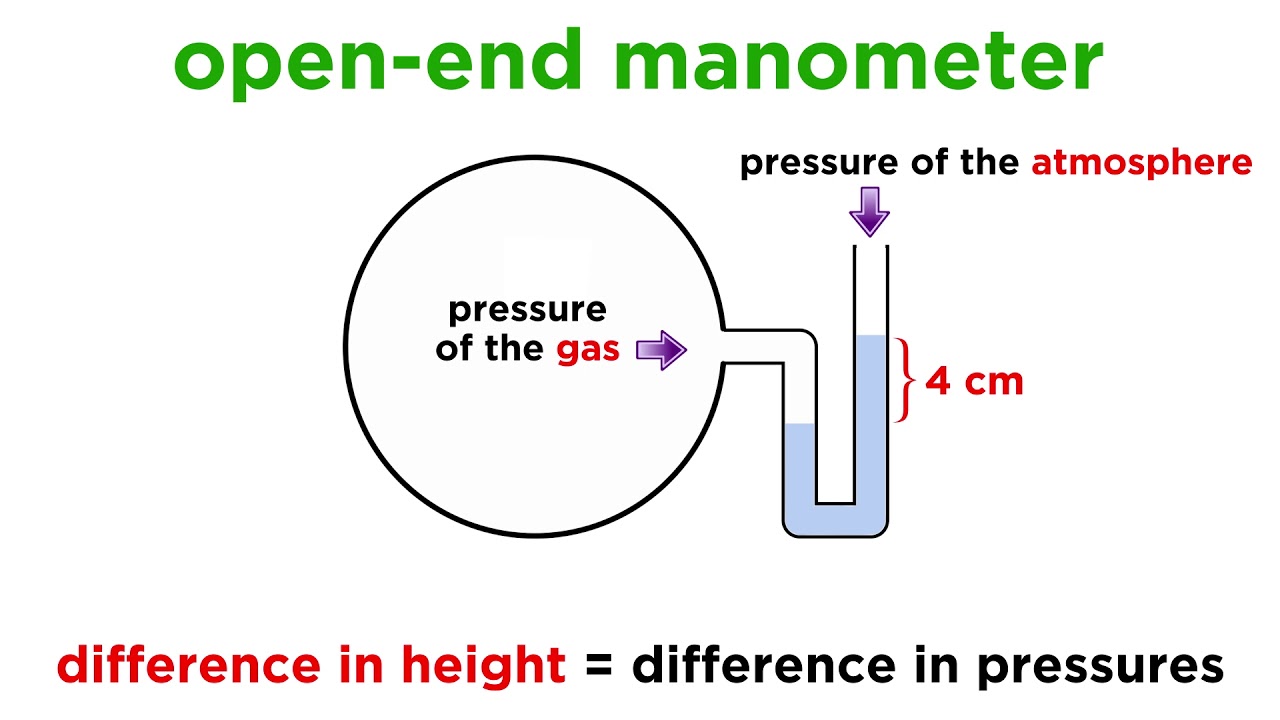
Which is greater, the pressure of the gas, or the pressure of the atmosphere?
the pressure of the gas
Describe Gay-Lussac's law of combining volumes.
The ratio of volumes of gases in a reaction is the same as the mole ratio (numbers in front of chemical formulas in the balanced equation).
A 6.0 L sample of gas contains 0.5 mole of a gas. If an additional 0.25 mole of gas at the same pressure and temperature are added, what is the final total volume of the gas?
V1/n1 = V1/n2
9 L
it would decrease by half
If the Ideal Gas Law is PV = nRT, what are the units for the constant R?
R = (L*atm)/(K*mol)
If the pressure of the atmosphere is 760 mmHg, what is the pressure of the gas?
10 cm = 100 mm
760 mmHg + 100 mmHg = 860 mmHg
What three variables are related in the Combined Gas Law?
Double the points if you write the formula!
pressure, volume, and temperature
(P1V1)/T1 = (P2V2)/T2
A sample of oxygen collected over water at 23°C has a total pressure of 743 torr. What is the partial pressure of the oxygen?
The vapor pressure of water at 23°C is 21.1 torr
721.9 torr
Name the two variables in Graham's law of diffusion.
rate of diffusion and molar mass of the gas
