molecular Forces
Atomic Radii
What is the distance from the nucleus of the atom to its outermost electron?
CS2
Cl in HCl
What is 0?
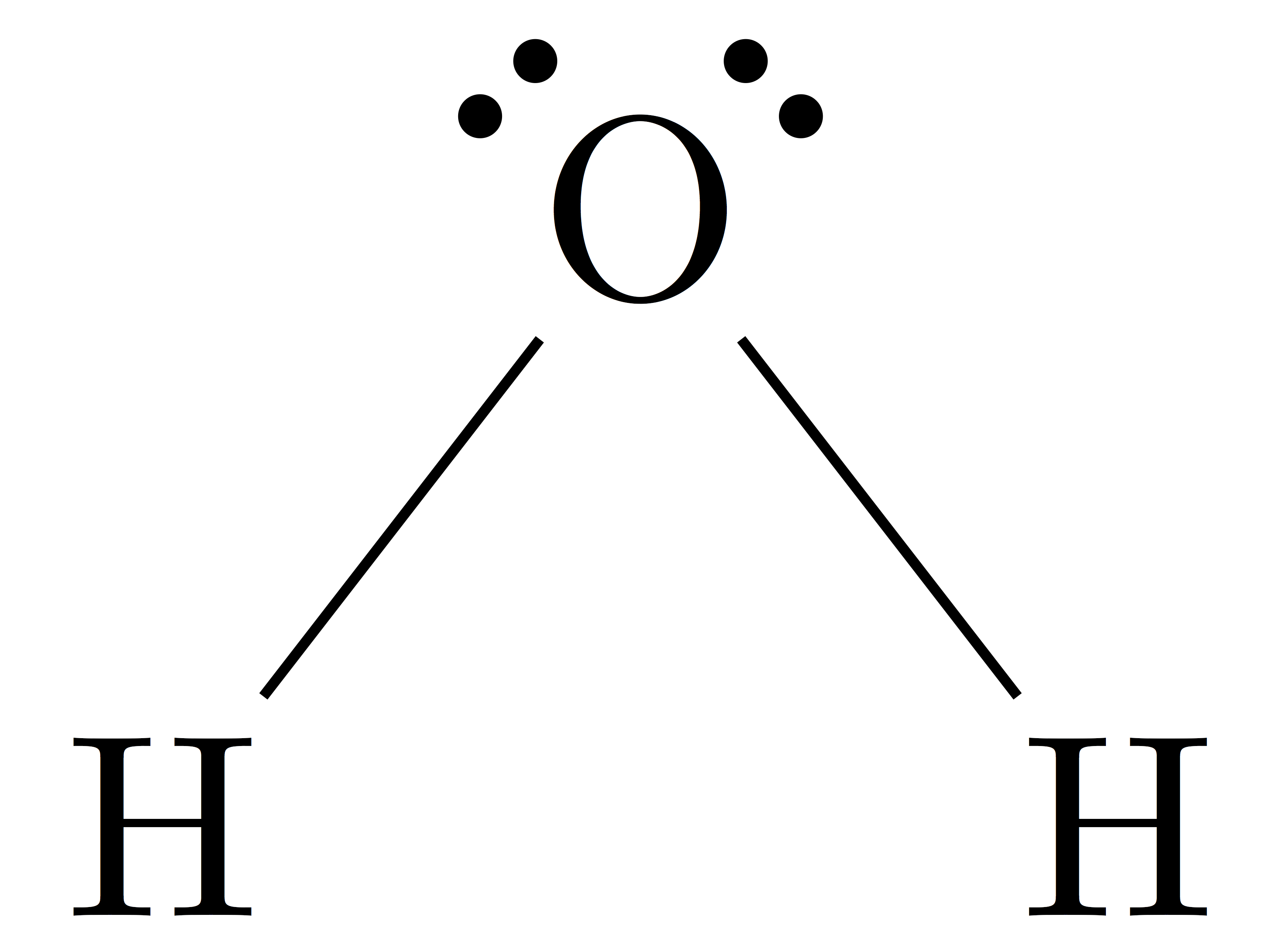
Bent
N2
What are London dispersion forces?
Ionization energy
What is the energy required to remove an electron from an atom?
NH4+
S in SO42-
What is +2?
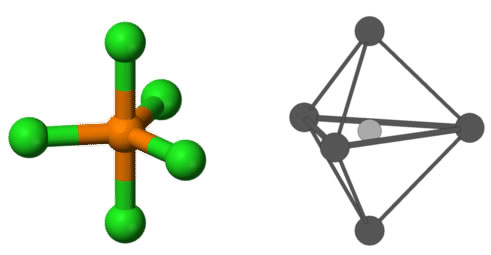
Trigonal bipyramidal
MgSO4
What are ion-dipole forces?
Energy change accompanying addition of an electron to gaseous atom
P in PO43-
What is +1?
FCl3
T-Shaped
NH3
What is a hydrogen bond?
This ion has a larger radii than its parent atom
What is an anion?
CH2O
Number of resonance structures of HCNO
What is 2?
SF4
See-Saw
Order from weakest intermolecular force between to strongest?
NH3, PH3, AsH3
PH3 < AsH3 < NH3
Groups that are exceptions to the ionization energy periodic trend
What is group 3A and 6A?
BeCl2


What is the first structure?
BrF5
Square Pyramidal
Has the highest boiling point
CH4, SiH4, GeH4, SnH4
What is SnH4?