This positively charged particle was discovered by Ernest Rutherford.
Who discovered protons?
The subatomic particle with no charge on it
neutron
who is Ernest Rutherford?

In the above figure, Element Y is added to element X. This makes it stronger, durable and hard to shift. what is this resultant structure/ process called?
it is called an alloy/ alloying
Double Jeopardy!
double points to be added
what is the range on the pH scale?
range is 0-14
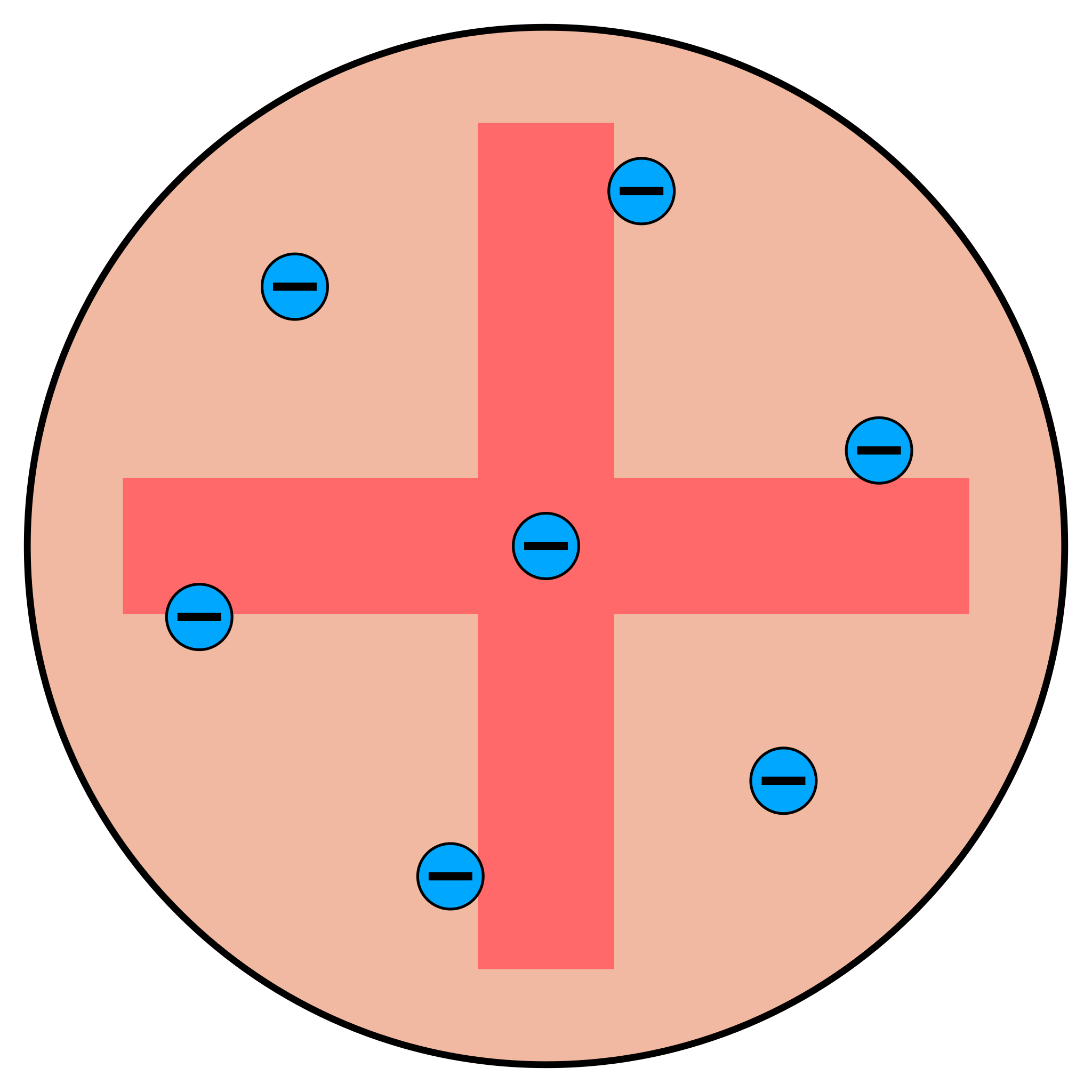
which model of the atom is represented here?
Plum pudding model
Neutral doublet was proposed by which scientist?
Ernest Rutherford
What are some reasons for choosing aluminium in a lot of daily use items like air planes, cooking vessels, bicycle bodies
it is durable
lightweight
malleable
stronger than a lot of metals and lightweight
What is an element?
a substance that is made up of just one type of materials/ atoms. has the same chemical composition throughout
List any three postulates/ statements from Dalton
atoms are indivisible
elements combine in whole numbers to form compounds
atoms of same elements have same properties
Paul Villard and Ernest Rutherford discovered these three rays while conducting experiments.
alpha particles, beta particles and gamma rays
This gas when passed through limewater, turn it milky. Which gas is it??
carbon dioxide
what is the % purity of 20K gold? ( you know the formula)
20/24x100=83.33%
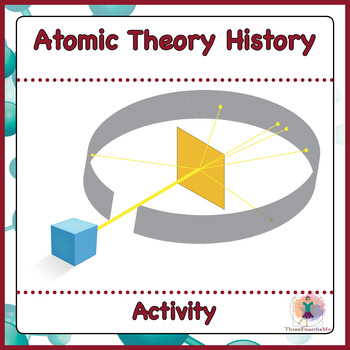
In the image given above:
what does the blue box represent?
The gray circle is the ..............
The yellow sheet is the ....................
blue box: alpha particle source/ emitter
gray circle: detector screen/ fluorescent screen
yellow sheet- gold foil