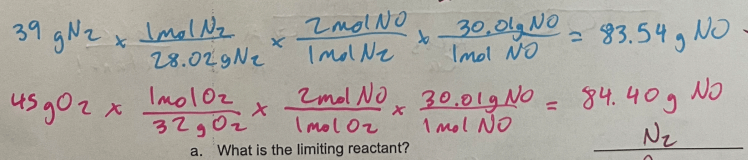(Stoich 1)
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
Using the balanced equation above, what are the possible mole ratios? Choose from the list below, there are multiple answers:
i. 2 KBr : H2SO4
ii. 2 KBr : HBr
iii. KBr : K2SO4
iv. 2 KBr : 2 HBr
v. K2SO4 : 2 HBr
i, iv, v
4 Fe + 3 O2 ->2 Fe2O3
If you have 7.5 mols of iron, how many mols of oxygen would be required?
5.63 mol O2
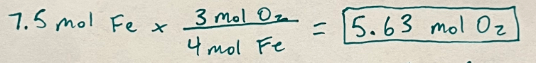
How many molecules of water do you have if you have 2.5 moles of water?
1.51 x 1024 molecules H2O
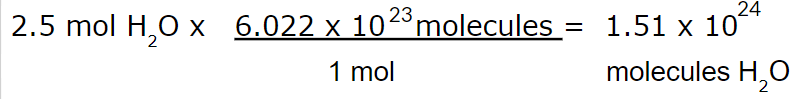
CH3COOH + NaHCO3 → NaCH3COO + H2O + CO2
A student reacts a solution of vinegar (clear liquid; CH3COOH) with baking soda (white powder; NaHCO3). When the reaction is finished, there is white powder at the bottom of the beaker. What is the limiting reactant?
a) CH3COOH
b) NaHCO3
c) NaCH3COO
d) H2O
e) CO2
a) CH3COOH
4 Fe + 3 O2 ->2 Fe2O3
Which of the following mole to mole ratios are correct for the reaction above? There are multiple answers.
i. Fe: O2
ii. 3 O2 : 2 Fe2O3
iii. Fe2O3 : 3 O2
iv. 4 Fe: 2 Fe2O3
ii and iv
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
If you have 25 moles of K2SO4 how many grams of H2SO4 will be produced?
2452.25 g H2SO4
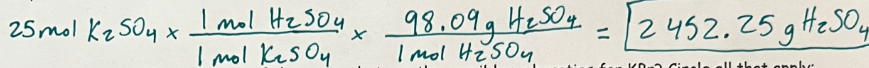
How many moles of compound Z is 2.37 x 1024 molecules of Z?
3.94 mol Z
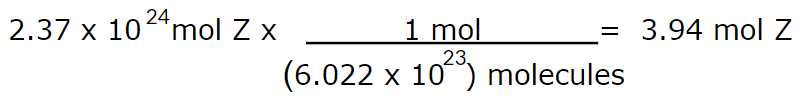
Ca + AlCl3 → CaCl2 + Al
A scientist conducts the reaction below to create calcium chloride and aluminum. They observe a small piece of calcium leftover and a small amount of aluminum left over. What is the excess reactant?
a) Ca
b) AlCl3
c) CaCl2
d) Al
a) Ca
You are burning a candle and you put the lid on the candle and the fire goes out. What is the limiting reactant?
Oxygen (O2)
2 K + Cl2 -> 2 KCl
If a student performs an experiment using 2.5 grams of chlorine, how many moles of potassium chloride will be formed?
0.07 mol KCl
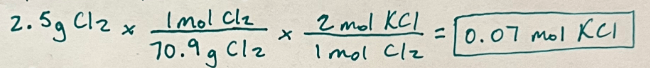
What is the percent composition by mass of oxygen in N2O3?
63.14 % O
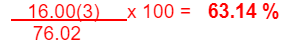
If 175 g of H2O decompose, how many molecules of H2O decomposed?
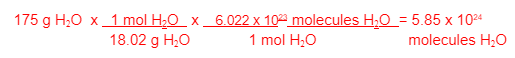
A piece of paper is set on fire outside and the fire eventually goes out. What is likely the limiting reactant of this reaction?
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
If you have 15 g of HBr, how many grams of KBr will be produced?
22.06 g KBr
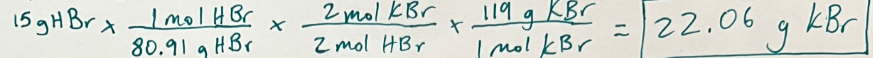
A new compound has been discovered with new elements. The compound formula is A3D5. Use the data table below to calculate the percent composition of element A in A3D5.
Molar Mass
A = 2.14
D = 1.49
46.3 % A
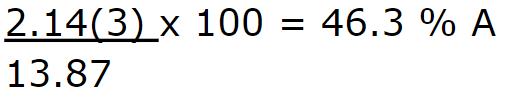
If you have 3.25 x 1022 molecules of C2H3O2, how many grams do you have?
3.19 g C2H3O2

A student’s hair catches on fire during a lab and the teacher throws the fire blanket on the student to put out the fire. What is the limiting reactant?
Oxygen (O2)
The following reaction is carried out by a student: Fe + Cl2 -> FeCl2
The student starts the reaction with 250 grams of iron. After the reaction is complete the student measures the mass of the FeCl2 that was produced. The student finds that 485 g of FeCl2 was produced. What is the percent yield for this experiment?
85.48%
Hexane, an example of an organic compound, is made up of 83.60% carbon and 16.40% hydrogen by mass. What is the empirical formula for this compound?
C3H7
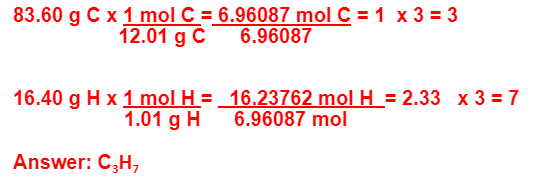
If you have 39 g N2 and 45 g O2 what is the limiting reactant?
N2 + O2 → 2 NO
N2 (produces smaller amount of product)