The processes of diffusion, osmosis and Brownian motion provide evidence for this theory.
PARTICULATE THEORY OF MATTER


List the three types of mixtures
solutions, colloids and suspensions
TRUE OR FALSE!
Homogeneous solutions cannot be separated.
FALSE
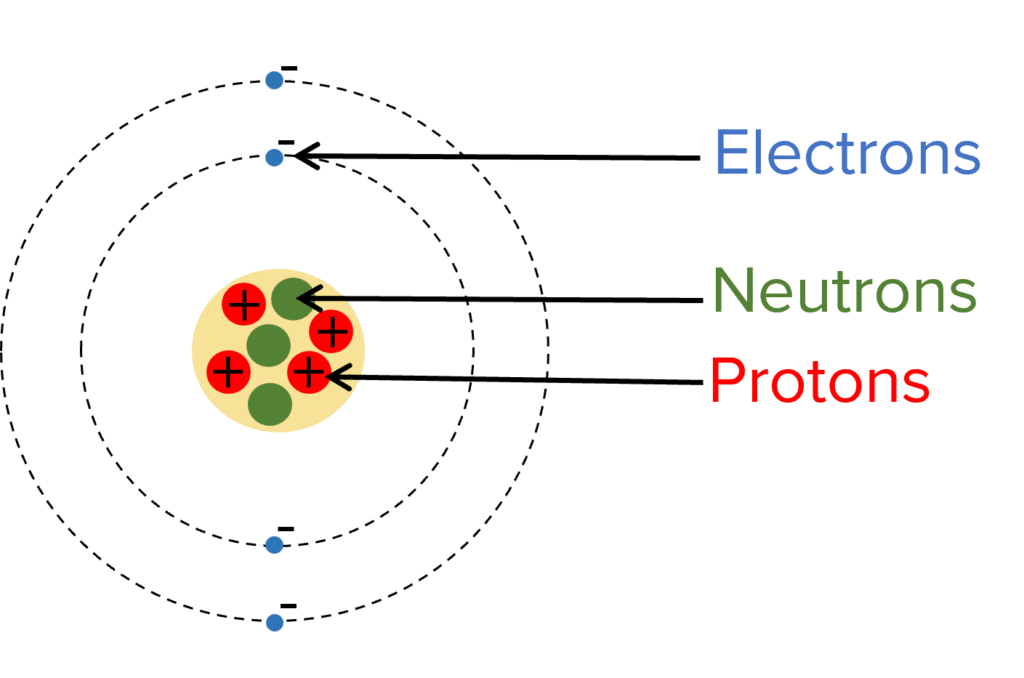
Label the subatomic particles present in an atom

(a) electron (b) neutrons (c) protons
Elements on the periodic table are arranged in order of increasing ________________ number.
Atomic Number
What are the names of the change of state processes?'

(A) melting (B) Boiling/Evaporation (C)freezing (D) condensation
Type of mixture where solute dissolves in a solvent
solution
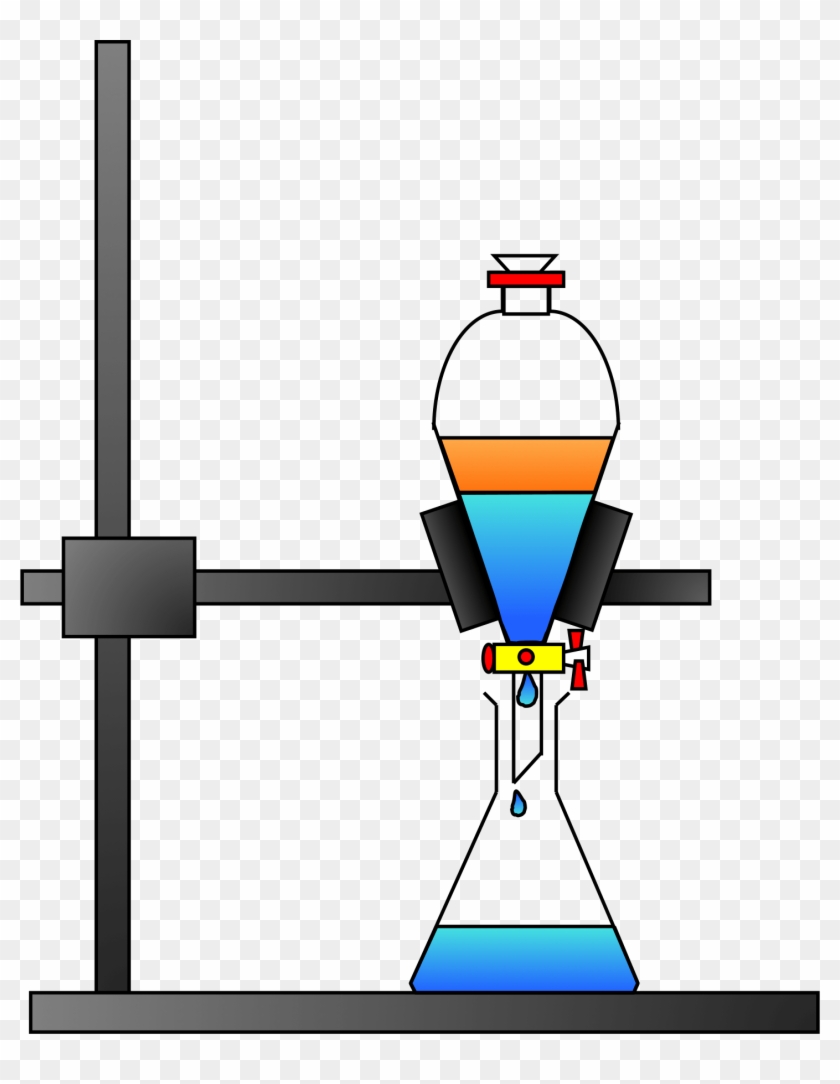
The diagram below shows the separating funnel used to separate immiscible mixtures of liquid. Provide an example of a mixture that can be separated using the separating funnel.

Calculate the number of protons, neutrons and electrons in the following atom

mass # = 19 and atomic # =9
Therefore,
# of protons = atomic number =9
# of neutrons = mass # - atomic # = 19 - 9 =20
# of electrons = # of protons =9
Recite the first twenty elements in the correct order.
Hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, silicon, phosphorus, sulphur, chlorine, argon, potassium , calcium
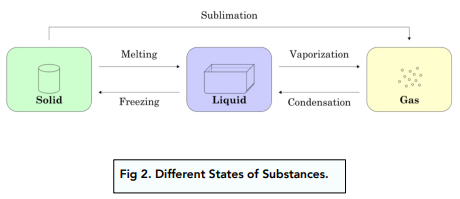
Which two diagrams show the states of matter before and after the sublimation of iodine?
(A) X to Y (B) X to Z (C) Z to X (D) Z to Y
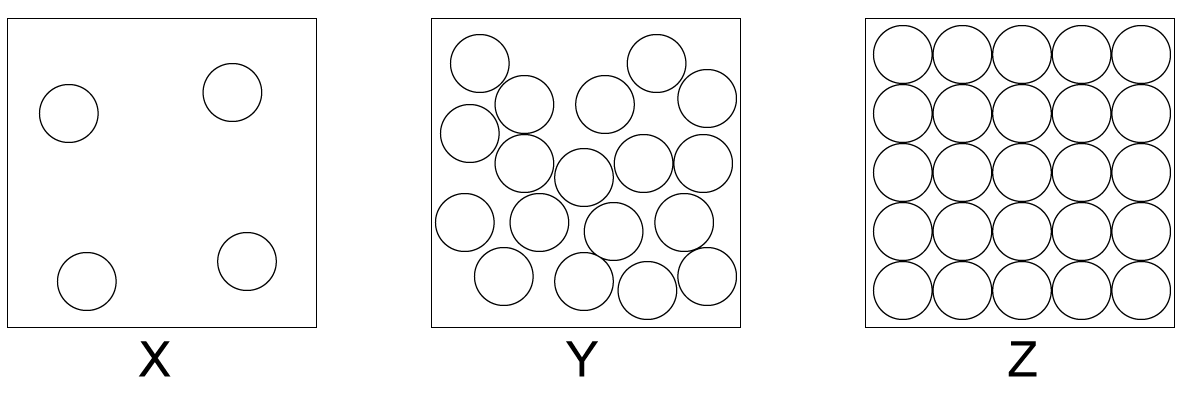
(C) Z to X. Sublimation involves the change from solid to gas
A heterogeneous mixture where the solute does not dissolve, but gets suspended in the solvent
SUSPENSION

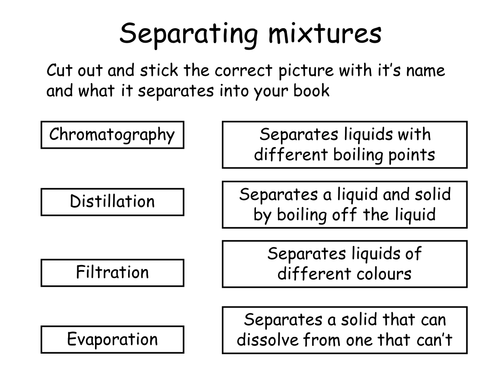
Match the following separation techniques to the types of mixture it is used to separate.
Chromatography - separates liquids of different colours
Distillation - separates liquids with different boiling points
Filtration - Separates a solid that can dissolve from one that can't
Evaporation - Separates a liquid and a solid by boiling off the liquid
Write the nuclear notation of the following atom that belong to element Z
74 Z
Mass number = 7 (because there are 4 protons and 3 neutrons)
Atomic Number = 4 (because there are 4 protons)
An Element Z has an electronic configuration 2, 8, 7. Place Element Z in its correct position on the Periodic Table.

Should be in Group VII and Period 3
Two equally sized potatoes were placed in different solutions and left for the same time. One was placed in distilled and the other placed in concentrated solution. What causes the potato strip in beaker 1 to increase in size and what causes the strip in beaker 2 to decrease in size ?
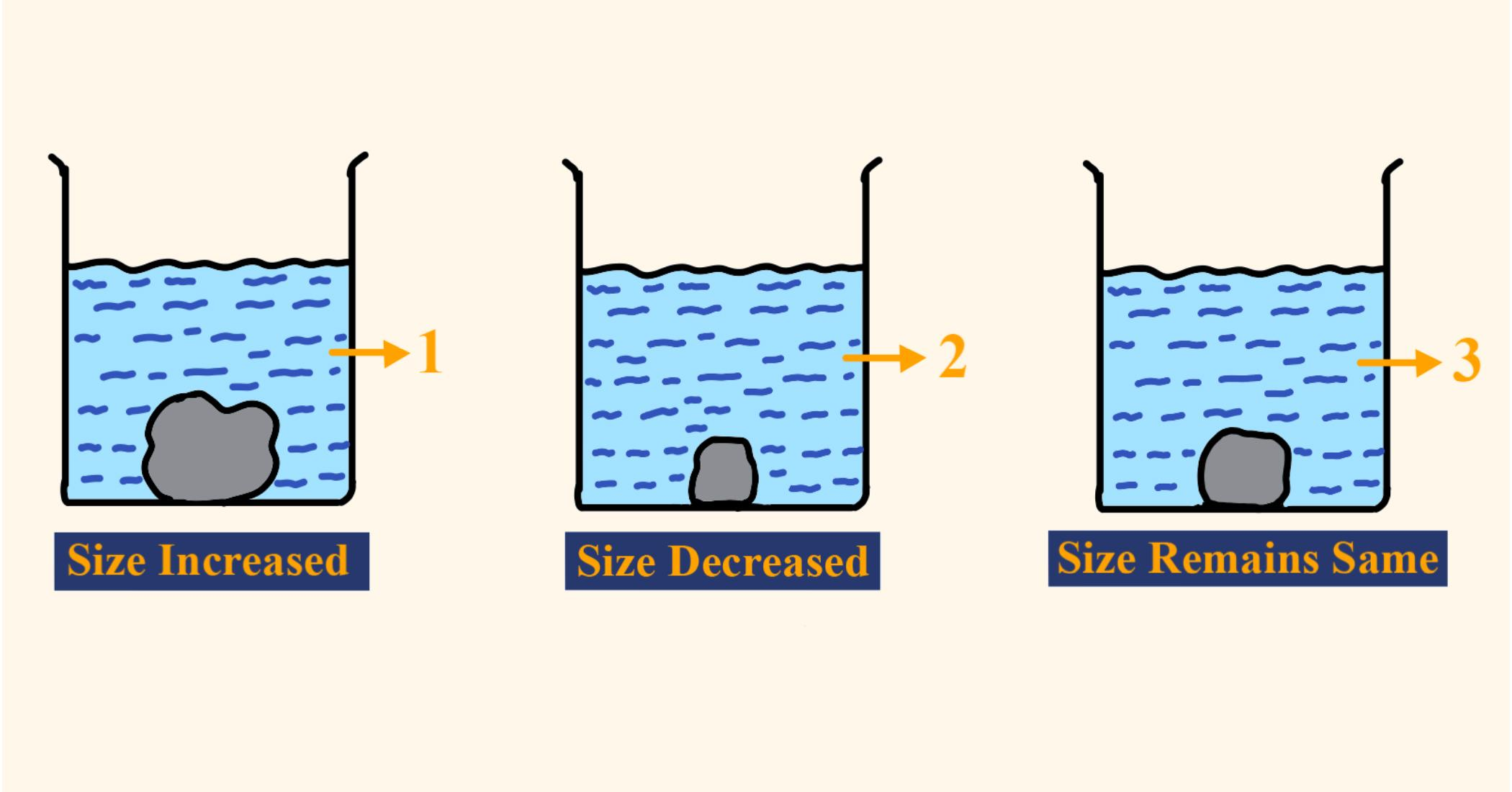
The cell of potato in beaker 1 increases in size because it absorbs water (since higher concentration of water is in the beaker than in the cell) while the cell of the potato in beaker 2 decreases in size because water moves out the cell of the potato (since higher concentration of water is in the cell than in the beaker)
Describe the three types of mixtures in terms of particle size
Solutions have particles which are invisible to the naked eye and invisible under a microscope (they have the smallest particles)
Colloids have particles which are invisible to the naked eye, but visible under a microscope (they have intermediate particles - larger than in a solution but smaller than in a suspension)
Colloids have particles which are visible to the naked eye (they have the largest particles)

Label the diagram below that shows the simple filtration of a mixture

From top left: beaker containing mixture, residue, filtrate
From to right: filter paper, filter funnel, conical flask
In what ways are the isotope of hydrogen similar and in what way are the isotopes different?
SIMILARITIES : (1) Same number of protons (2)Same number of electrons (3) All atoms have an overall charge of zero (4) Similar chemical properties
DIFFERENCES: (1) Different number of neutrons (2)Different mass number (3) The nucleus of hydrogen-1 is more stable than hydrogen-2 and hydrogen-3 (4) Different physical properties
What is the electronic configuration of an element X which is found in Group IV and Period 3?
2,8,4
What is the boiling point of the following substance?
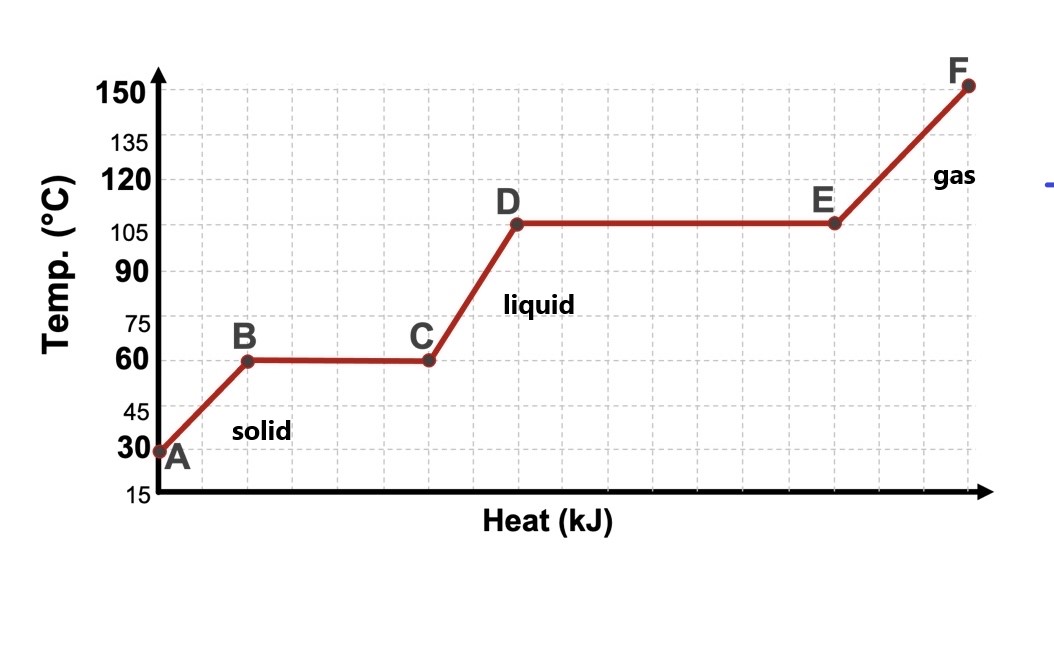
105oC
What is the solubility of KNO3 at 700C?

100 g/100g H2O
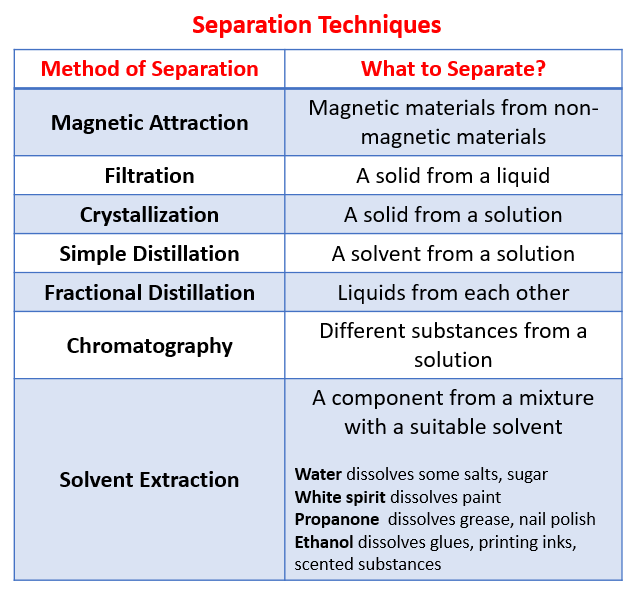
State two similarities and two differences between the separation techniques of simple distillation and fractional distillation.


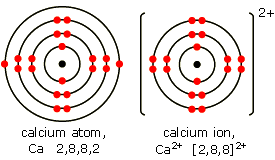
Draw the structure of an ion of calcium

An ION of particular element carries a charge of +1. In which family group of Periodic Table will the element belong?
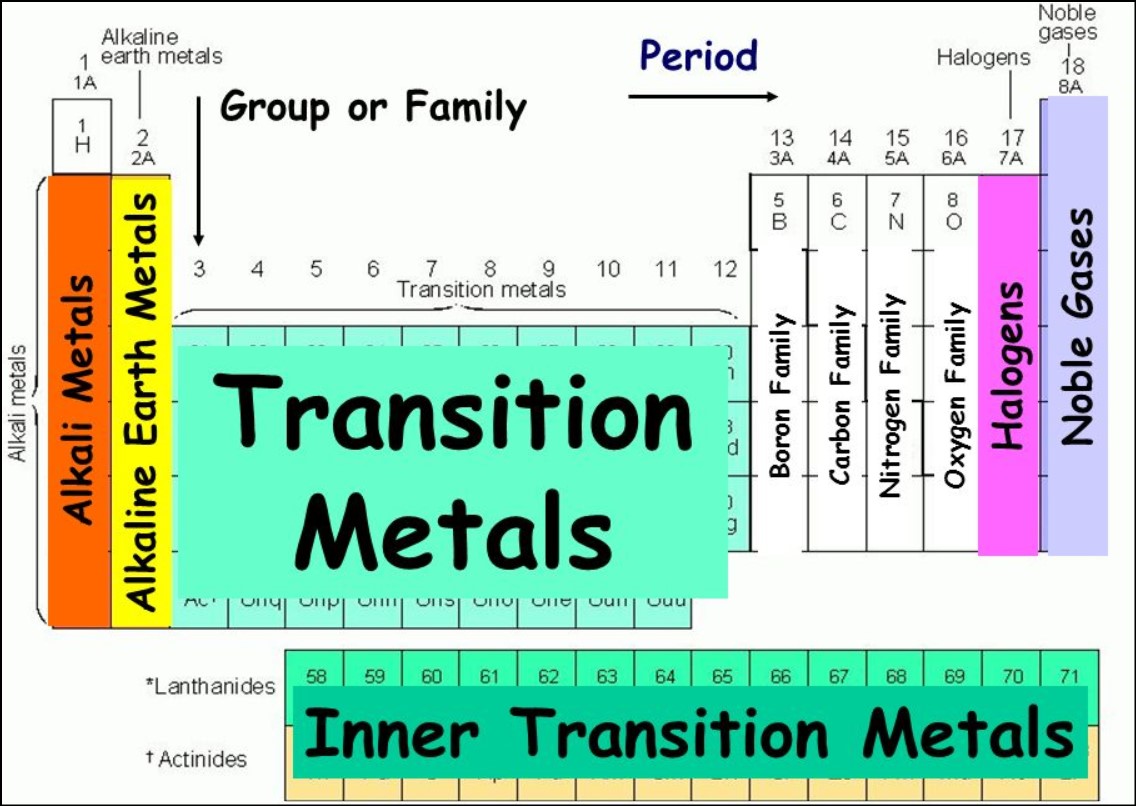
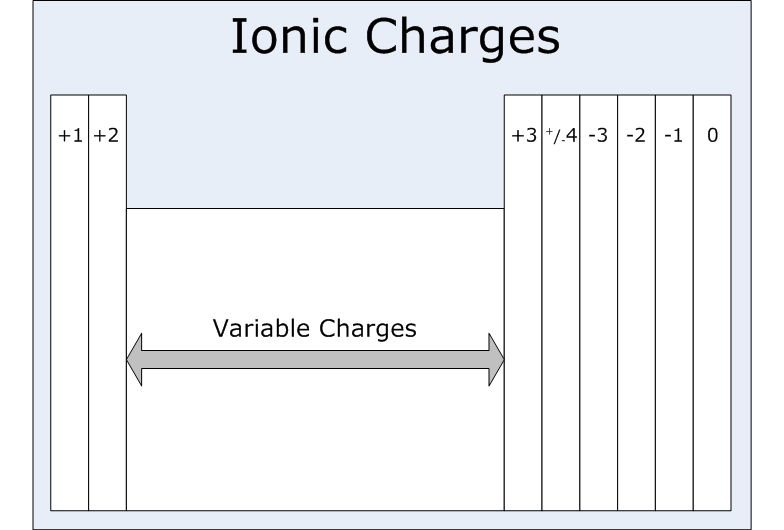 An ion which has a +1 charge would belong to group I and is likely an alkali metal.
An ion which has a +1 charge would belong to group I and is likely an alkali metal.