Write down the double replacement reaction between iron (III) bromide (aq) and sodium
hydroxide (aq). If 12 grams of both compounds are reacted, how many moles of product can we
expect to form? Which one is the limiting reagent? Return to your General Chemistry solubility rules
as we will only form one solid product.
0.0406 mol Fe(OH)3
is produced; FeBr3
is the LR.
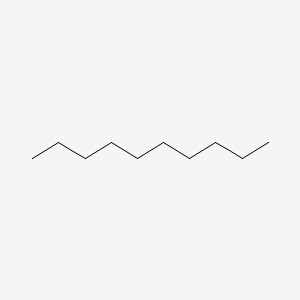
Which molecule has the higher boiling point?
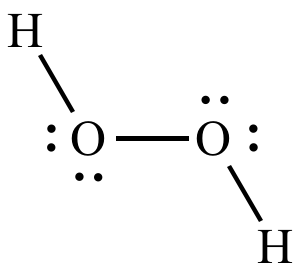
What is the volume of one mole of gas inside a container under STP conditions?
22.4 L
Atoms of which element have the smallest ionization energy?
-Na, F, K, Cl
K
What are the names of your peer leaders (first and last)?
Anthony Aragon
Ashleigh Min
Draw the Correct Lewis structure of Chromate ion
CrO4
Ethanol has a density of 0.789 g/mL at 25 C. Calculate the molality of a 4.86M ethanol solution at this temperature.
8.6m
endothermic that forms sugar or an exothermic that forms sugars
An endothermic reaction that forms sugars
Which metal has the highest melting point?
K, Ca, Fe, Zn
Fe
What is Anthony/ Ashleigh's major?
Anthony : Biomedical Science
Ashleigh : Biology
What is the hybridization of Boron in Borate, and what is the Molecular geometry
SP2 Trigonal Planar
Determine the overall order of the reaction. What is the value of the rate constant k?
Experiment 1:[HgCl2] (M): 0.105, [C2O42-] (M): 0.15, Initial Rate (M/min): 1.8x10-5
Experiment 2:[HgCl2] (M): 0.105, [C2O42-] (M): 0.15, Initial Rate (M/min): 1.8x10-5
Experiment 3:[HgCl2] (M): 0.052, [C2O42-] (M): 0.30, Initial Rate (M/min): 7.1x10-5
Experiment 4: [HgCl2] (M): 0.052, [C2O42-] (M): 0.15, Initial Rate (M/min): 8.9x10-6
[HgCl2]: 1st
[C2O42-]: 2nd
Overall Order: 3rd
k= 7.6x10^-3 1/M^2min
An orbital in a ground-state gas-phase As atom has n=3, l=1. How many electrons are in this orbital?
2
How many pi bonds are in a molecule of propane, C3H4?
2
What does Dr. Narayan study in his research lab?
How many SP2 Hybridized Carbons in Meta Diethyl Benzene?
6
Copper(I) oxide, Cu2O, is reduced to metallic copper by heating in a stream of hydrogen gas. What mass of water is produced when 10g copper is formed?
1.417 g
What is the electron configuration of Al3+ ion?
1s22s22p6
In the Lewis structure of the chlorate ion, how many lone pairs of electrons does the chlorine atom have?
1
What was Dr. Becvars childhood nickname?
The molecule H2N-CH2-CO-NH-CH2-O-CH2-CHOH-CH2-CO2H contains all of the functional groups except: carboxylic acid, primary amine, amide, ether, alcohol, carbonyl, ketone
Ketone
The mineral energize is 48.41% Cu, 19.02% As, and 32.57% S by mass. What is the empirical formula of energite?
Cu3AsS4
The wavelength of one line in the emission spectrum of C is 538 nm. What is the energy of one photon with this wavelength?
3.69E-19 J
In the [C(NH2)3]+, what is the best description of the hybridization of the nitrogen atoms?
All three sp2
Name all of the Chem 1306 Peerleaders. (clue there are 31!)
Ashleigh Min
Anthony Aragon
William Willars
Joshua Cayme
Christopher Trejo
Karim Garciayala
Paola Barron
Georgina Bugarini
Salma Saenz
Alejandro Padilla
Ashley Villarreal
Austin Blake
Carlos Chihuahua
Carlos M
Christina Valtierra
Enrique Bermudez
Joe Salazar
Johnathan Lopez
Jose Lopez
Mauricio Moreno
Natalia Garcia
Noah De La Torre
Paulina Trevino
Samantha Zepeda
Sophia Borrego
Kutaiebah Soueidan
Valeria Silva
Sebastian Garcia