Why are HCP metals typically brittle?
Although they are close packed structures, they have a small number of slip systems (3 systems).
How many solid phases do you expect to see on an isomorphous binary phase diagram?
one
What are the two stages of a phase transformation
nucleation and growth
What is the main difference between thermoset vs thermoplastic?
Thermoplastics
Soften when heated (and eventually liquefy) and harden when cooled—processes that are totally reversible and may be repeated. Mostly linear with some branching in structure.
Thermosetting polymers are network polymers. They become permanently hard during their formation and do not soften upon heating. Network polymers have covalent crosslinks between adjacent molecular chains. During heat treatments, these bonds anchor the chains together to resist the vibrational and rotational chain motions at high temperatures. Thus, the materials do not soften when heated.
What is the principle of combined action?
Combine materials with the objective of getting a more desirable combination of properties
How does adding smaller impurity atoms strengthen a metal?
Relaxes the compressive strain field around a dislocation and thus more energy is required for the dislocation to move in response to a shear stress
A 90 wt% Ag–10 wt% Cu alloy is heated to a temperature within the β + liquid phase region. If the composition of the liquid phase is 85 wt% Ag, determine
(a) the temperature of the alloy
(b) the composition of the β phase
(c) the mass fractions of both phases
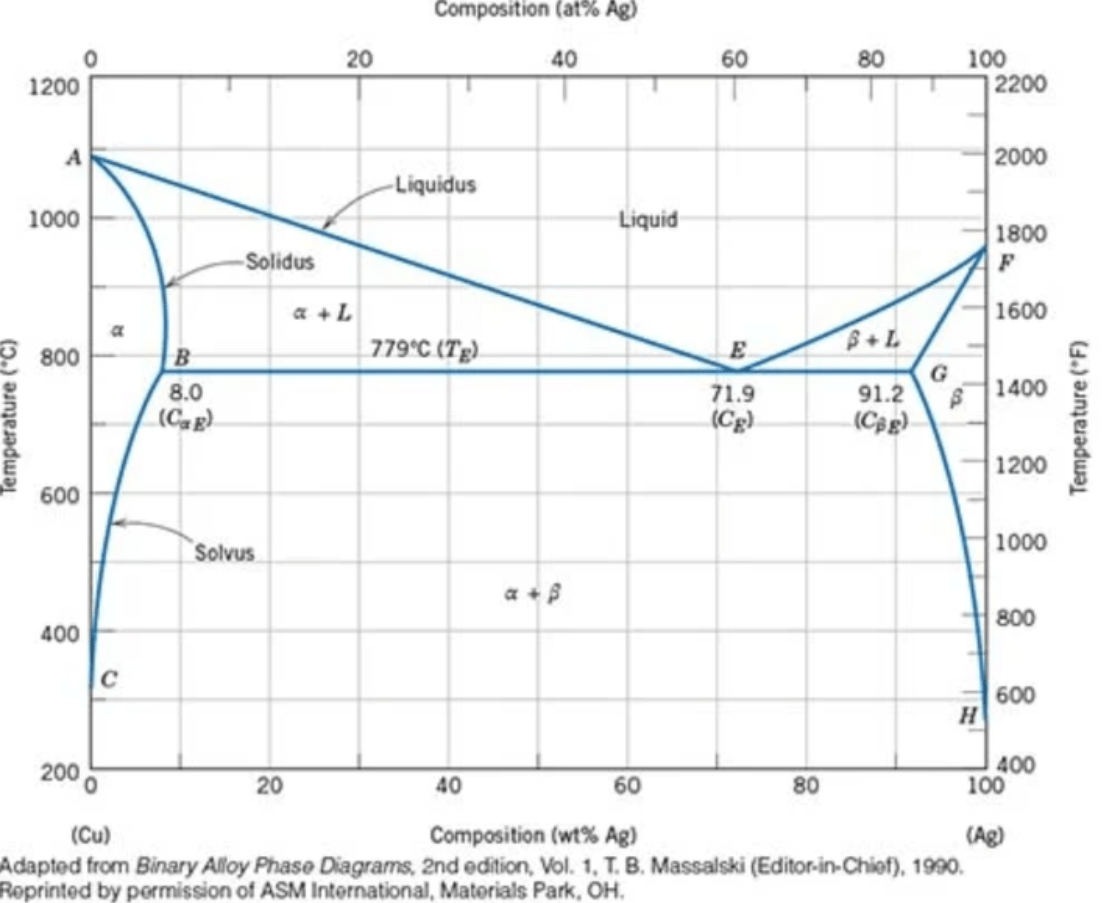
a) ~850 C
b) ~95 wt% Ag
c) roughly half of each phase
Say you have a specimen that is 100% austenite. It is quickly quenched to room temperature and transforms completely to martensite. Is this a diffusion dominated process? Why or why not?
Diffusionless. Not enough time for atoms to migrate. Atoms have only slightly shifted in position (Body centered tetragonal).
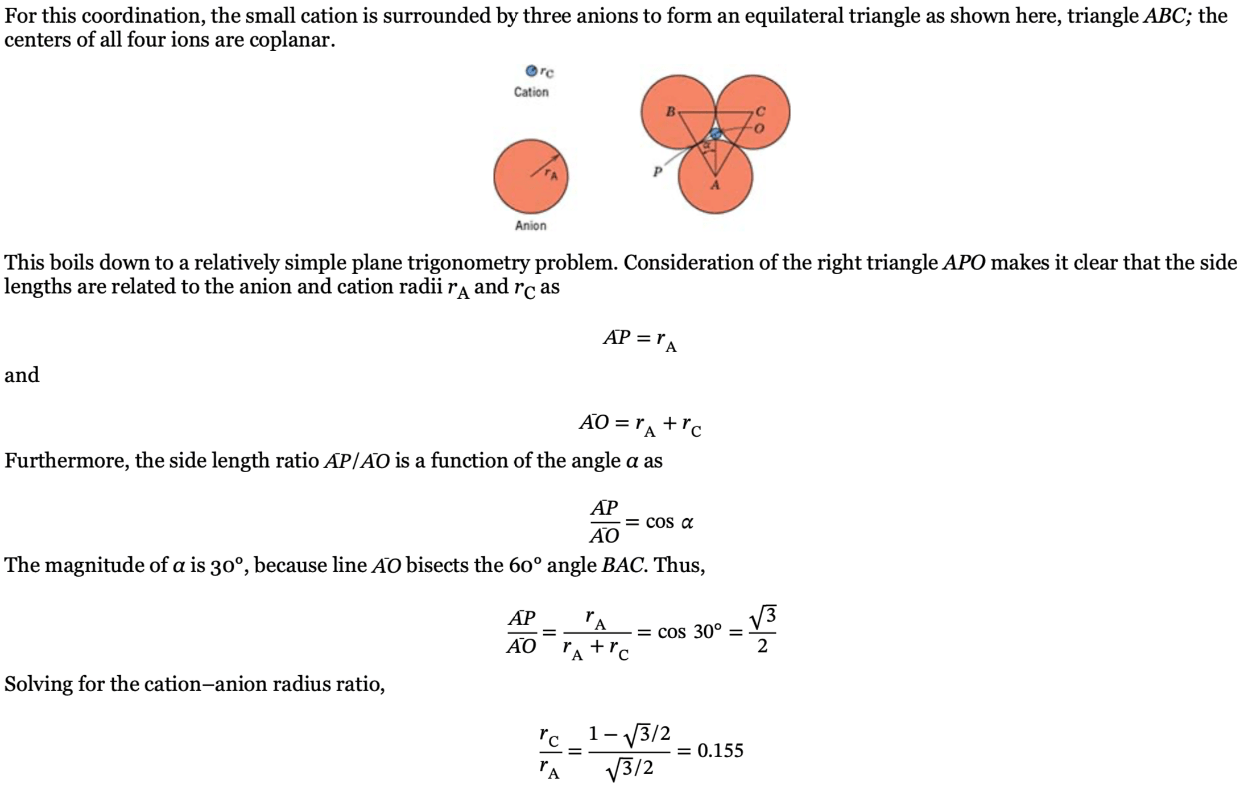
What does the ratio of the cation to anion radius tell us about the structure
Tells us about the coordination geometry (linear, triangle, tetrahedral, octahedral, cubic)
Give an example of a particle reinforced composite.
spheriodite, tungsten carbide and cobalt, commercial tires (carbon black in latex), concrete (sand, gravel and cement)
Explain the differences in the fracture surfaces for aluminum vs gray cast iron

For the cup and cone fracture, after necking begins, small cavities, or microvoids, form in the interior of the cross section. As deformation continues, these microvoids get larger and coalesce to form an elliptical crack. Finally, fracture ensues by the rapid propagation of a crack around the outer perimeter of the neck by shear deformation at an angle of about 45° with the tensile axis—the angle at which the shear stress is a maximum.
For the brittle material, fracture happens without plastic deformation.
What kind of information can we NOT obtain from an equilibrium phase diagram
kinetics of phase transformations including metastable phases
What are microconstituents present for specimen with a eutectic composition that has been subjected to the following time–temperature treatment? Assume that the specimen has been held long enough to have achieved a complete and homogeneous austenitic structure.
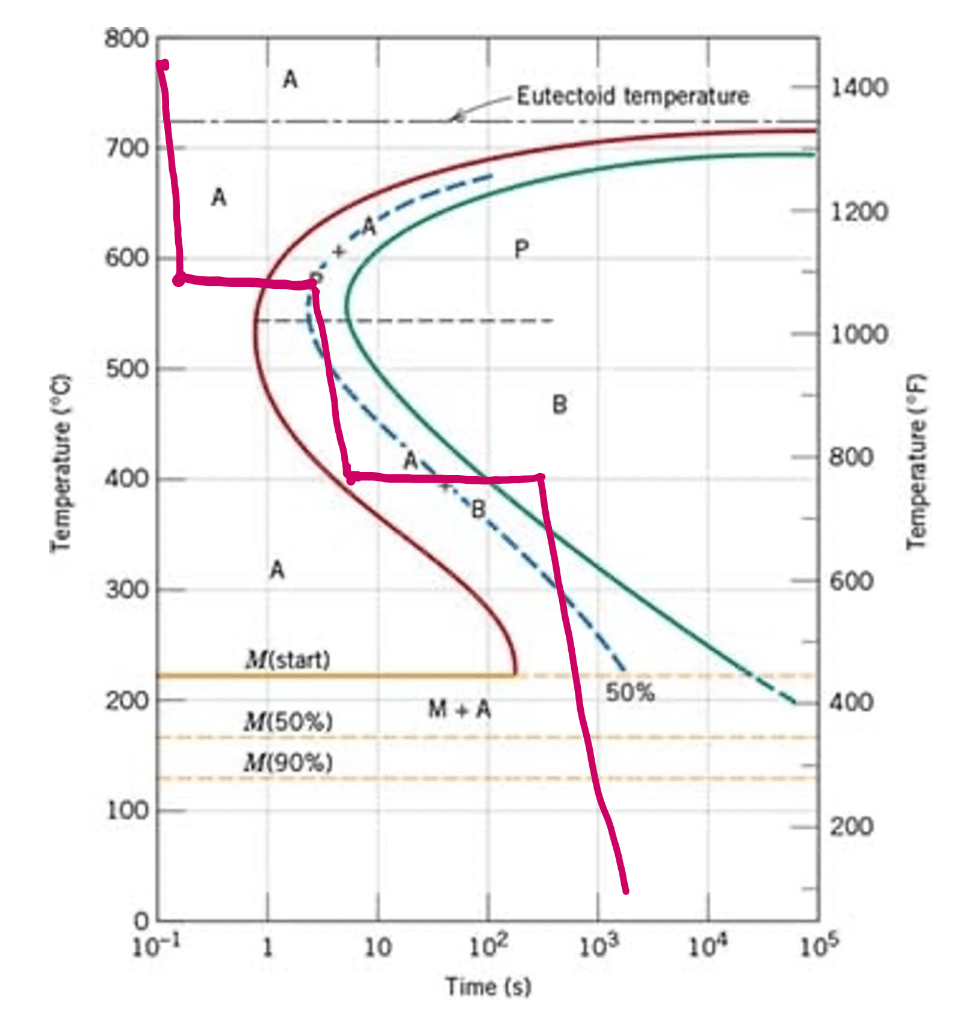
Fine pearlite and Bainite
a) Determine the number of cations and anions in this unit cell
b) determine the coordination number of the cation and anion
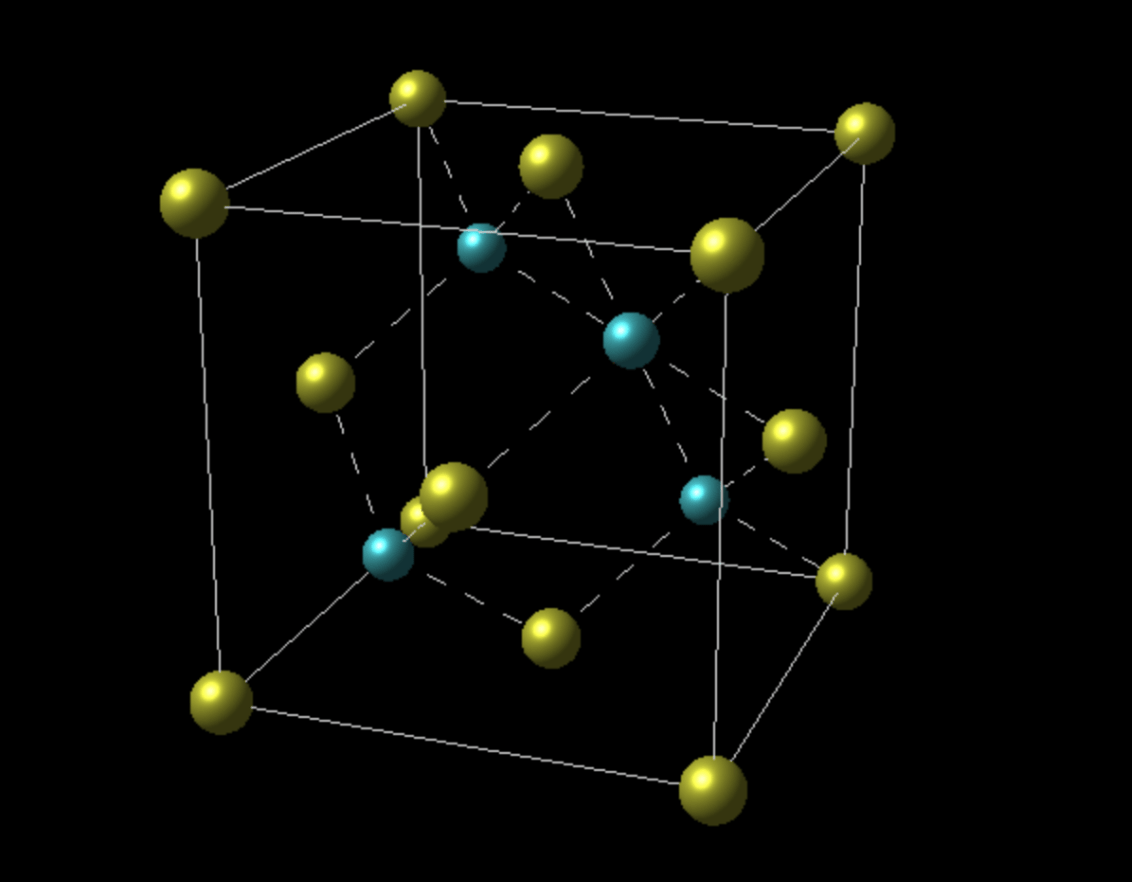
a) 4 cations and 4 anions per unit cell
b) coordination number = 4
Why does the conductivity of a conductor go down with increase in temperature?
reduces carrier mobility because there is more scattering at higher temperatures
a) What is fatigue limit
b) which materials shown on the graph have a fatigue limit?
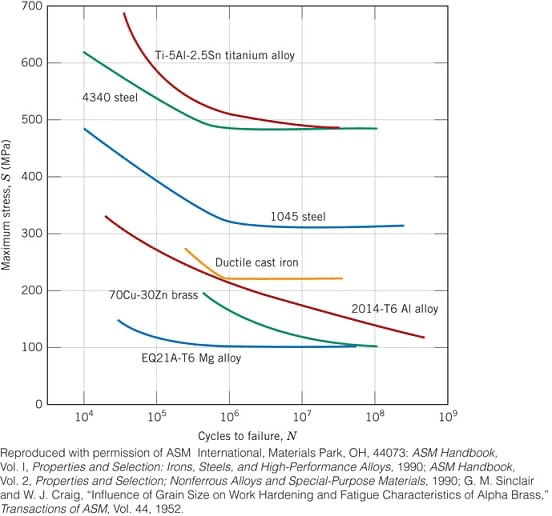
a) A limiting stress level, where below it fatigue failure will not occur. This fatigue limit represents the largest value of fluctuating stress that will not cause failure for essentially an "infinite" number of cycles
b) both steels, ductile cast iron, Mg alloy
What is the difference between a cored structure vs an equilibrium structure for a Cu-Ni system with 35 wt% Ni?
Equilibrium structures are obtained when cooled from the liquid phase to solid phase slowly enough so that atoms have sufficient time to migrate achieve a uniform structure of 35 wt% Ni throughout.
A cored structure produces layers that vary in composition, with the first solid phase to contain a higher wt% of Ni (~46 wt%) and the very last layer of solid to contain less than 35 wt% Ni.
What are the microconstituents present of a specimen that has been subjected to the following time–temperature treatment? Assume that the specimen begins at 760°C and that it has been held at this temperature long enough to have achieved a complete and homogeneous austenitic structure.
Rapidly cool to 575°C, hold for 20 s, rapidly cool to 350°C, hold for 100 s, then quench to room temperature.
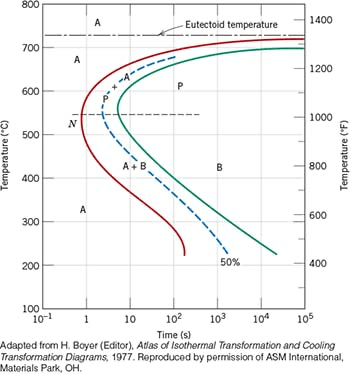
100% fine pearlite
DAILY DOUBLE:
Show that the minimum cation–anion radius ratio for the coordination number 3 is 0.155
What is the difference between p-type and n-type semiconductors?
create free charge carriers (electrons or holes depending on the electronic structure of the dopant)
Boron doped in silicon will create positively charged carriers (called holes) that can move around - p-type
Phosphorous doped in silicon will create free electrons - n type
note: silicon itself is an intrinsic semiconductor but is commonly doped for most applications.
What is the relationship between the yield strength of a single crystal and the critically resolved shear stress and the orientation of the most favorably oriented slip system?
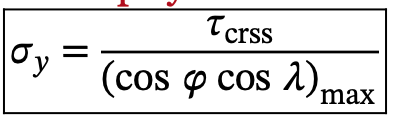
When phi and lambda = 45 degrees:
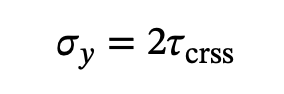
a) Using this diagram, briefly explain how spreading salt on ice that is at a temperature below 0°C (32°F) can cause the ice to melt.
b) At what temperature is salt no longer useful in causing ice to melt?
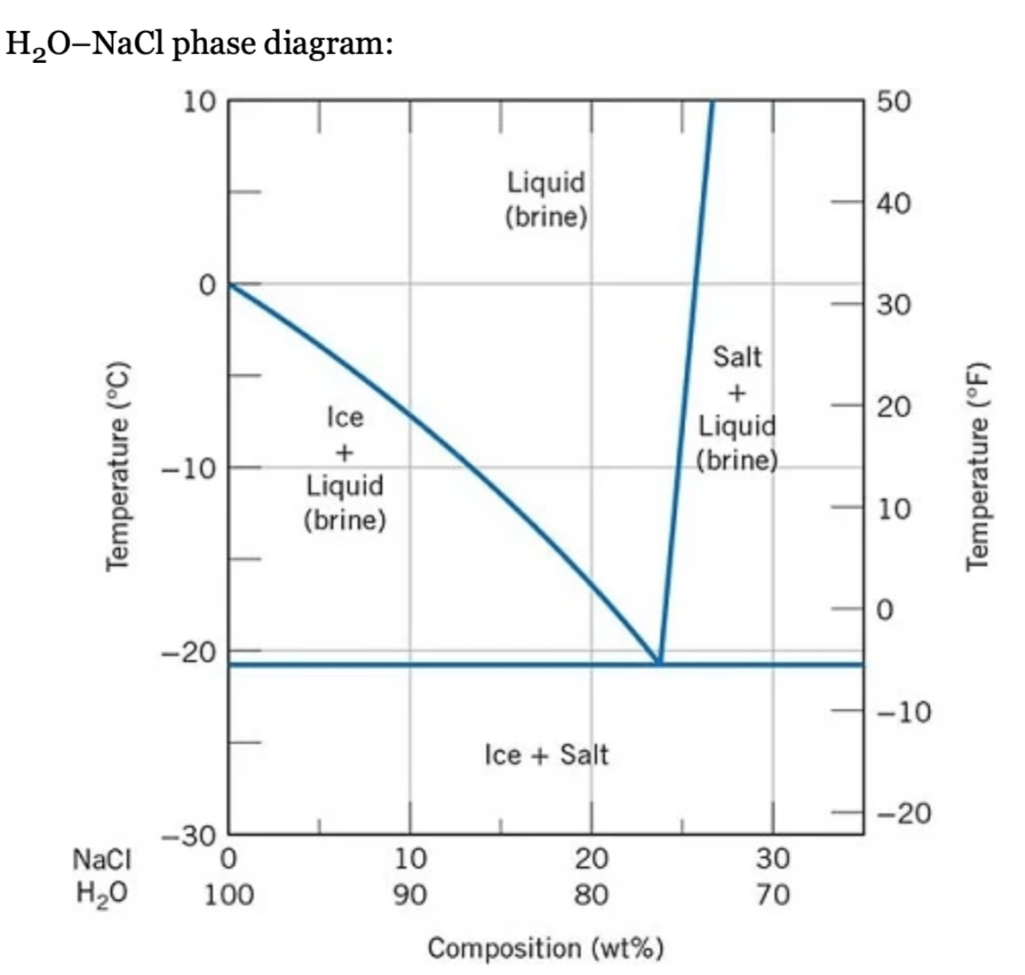
a) Spreading salt on ice will lower the melting temperature, since the liquidus line decreases from 0°C (at 100% H20) to the eutectic temperature at about −21°C (23 wt% NaCl). Thus, ice at a temperature below 0°C (and above −21°C) can be made to form a liquid phase by the addition of salt.
b) At −21°C and below ice is no longer useful in causing ice to melt because this is the lowest temperature at which a liquid phase forms (i.e., it is the eutectic temperature for this system).
What microconstituents would you observe for a 1.1 wt% c steel alloy specimen that has been quenched from 900 °C to 680 °C and held isothermally for 10 seconds before quenching to room temperature?
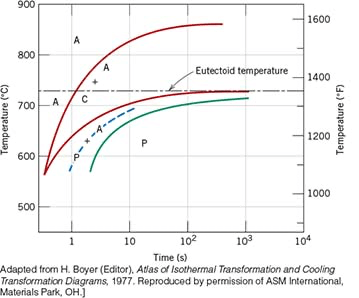
Proeutectoid cementite, coarse pearlite and martensite
DAILY DOUBLE:
Why are ceramics brittle?
Dislocation is unlikely in ionic crystals because of their positive and negative ions. For a given crystallographic plane, when atoms start to glide, the cations will repel each other as well as the anions.
For covalent bonds, such as diamond, these bonds are very strong and thus the material is hard. Hardness is the ability to resist localized plastic deformation.
a) What are four benefits of using carbon fiber reinforced plastics for commercial airplanes?
b) What is a limitation of using CRFP
a) Lightweight - fuel efficient
Durable - less maintenance required
chemically inert - corrosion resistant
Low coefficient of thermal expansion - does not expand or contract with dramatic changes in temperature during take off.
b) cost, complicated modeling (simulations), not good for lightning strikes