What is the positive part of the atom called?
a) Neutron b) Electron c) Proton
c) Proton
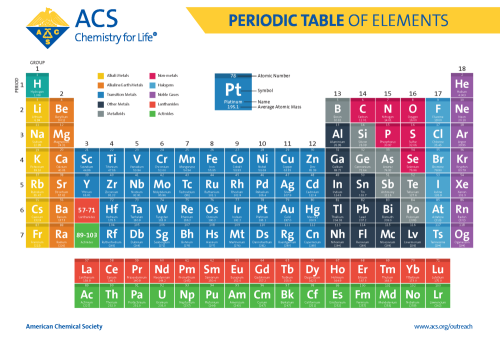
What side of the periodic table are the non-metals found?
The right side (+ hydrogen)
Universal indicator is added to vinegar (an acid), what colour will it turn?

Orange or red
Name any reactants in this equation.
Sodium + water --> sodium hydroxide + hydrogen

Sodium and water
What is an atoms goal in life? AKA why does it lose or gain electrons to become an ion?

To get a full outershell of electrons, so it can be stable.
What type of change is going on here? Physical or chemical. How do you know?
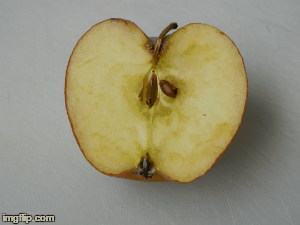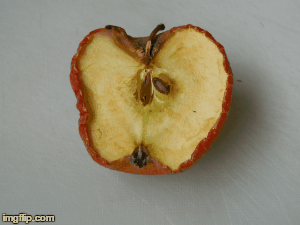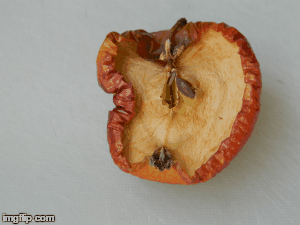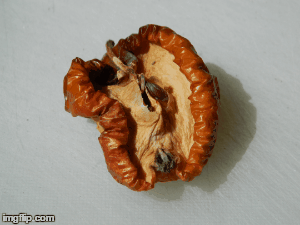
Chemical - it is not reversible, the apple will change in taste and smell, because its chemical make up is changing.
1. What type of ions does a base have more of?
2. What type of ions does an acid have more of?
OH- (hydroxide ions) or H+ (hydrogen ions)
1. Hydroxide ions: OH-
2. Hydrogen ions: H+

What type of reaction has occurred if an acid and a base are added to each other until they balance each other out.
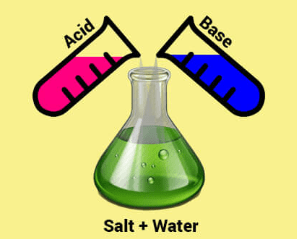
A neutralisation reaction. The H+ ions from the acid react with the OH- ions from the base to form H2O, which is neutral.
How many protons, electrons and neutrons does Aluminium have?
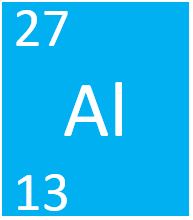
Atomic number = number of protons = number of electrons (for an atom)
Mass number - atomic number = number of neutrons.
13 Protons, 14 Neutrons, 13 Electrons.
What type of change is going on here? Chemical or physical? Explain how you know.

Physical - nothing new is being made, sugar is being mixed into coffee, but it is still sugar and coffee. The two are not reacting to make something different.
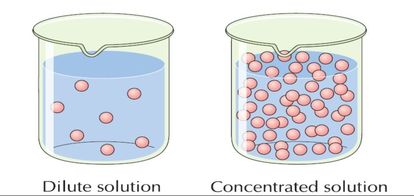
Draw what a concentrated solution looks like, compared to a dilute solution.
What has to happen between particles for a reaction to happen?
They must collide with each other
Magnesium is in group 2 and period 3, what does that tell us about Magnesium?
%20Periodic%20groups%20and%20periods.jpg)
Group 2 = Magnesium has 2 valence electrons
Period 3 = Magnesium has 3 electron shells

Which of these are characteristics of a non-metal?
a) Shiny
b) Bad conductor of heat and electricity
c) Brittle (breaks easily)
d) Can be turned into wire
e) Dull (not shiny)
b, c and e (Bad conductor, brittle and dull)

Which of these shows a weak acid?
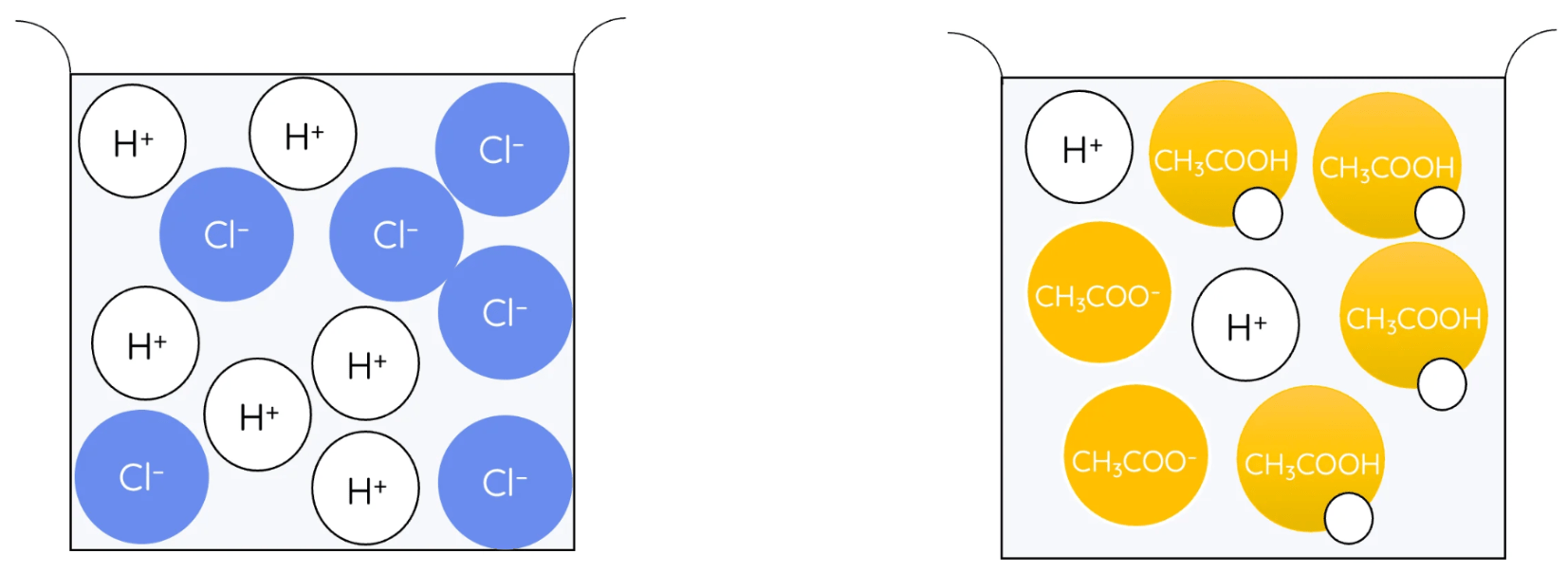
The one on the right which only some of the H+ ions have separated from.
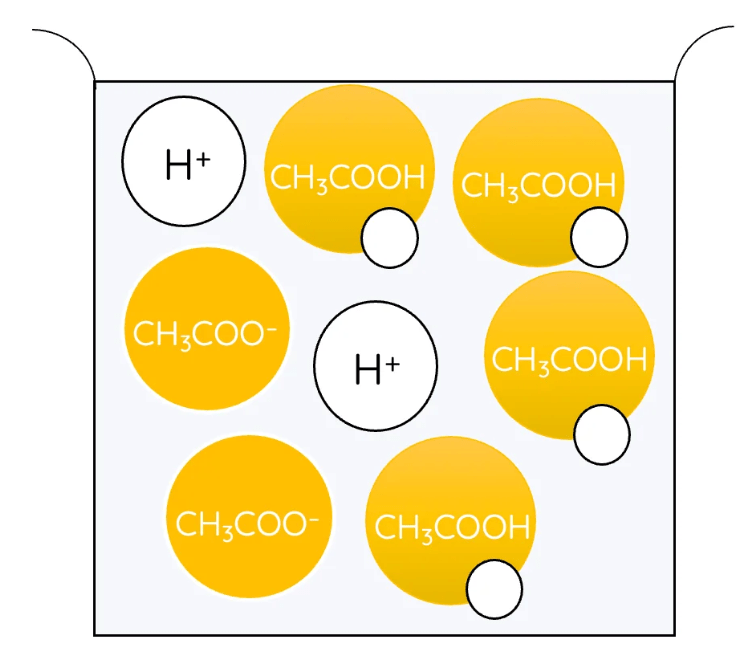
If you slowly add sodium hydroxide (base) to hydrochloric acid (acid) in a neutralisation reaction, what will happen to its pH? What colour change would you notice if it had universal indicator in it?
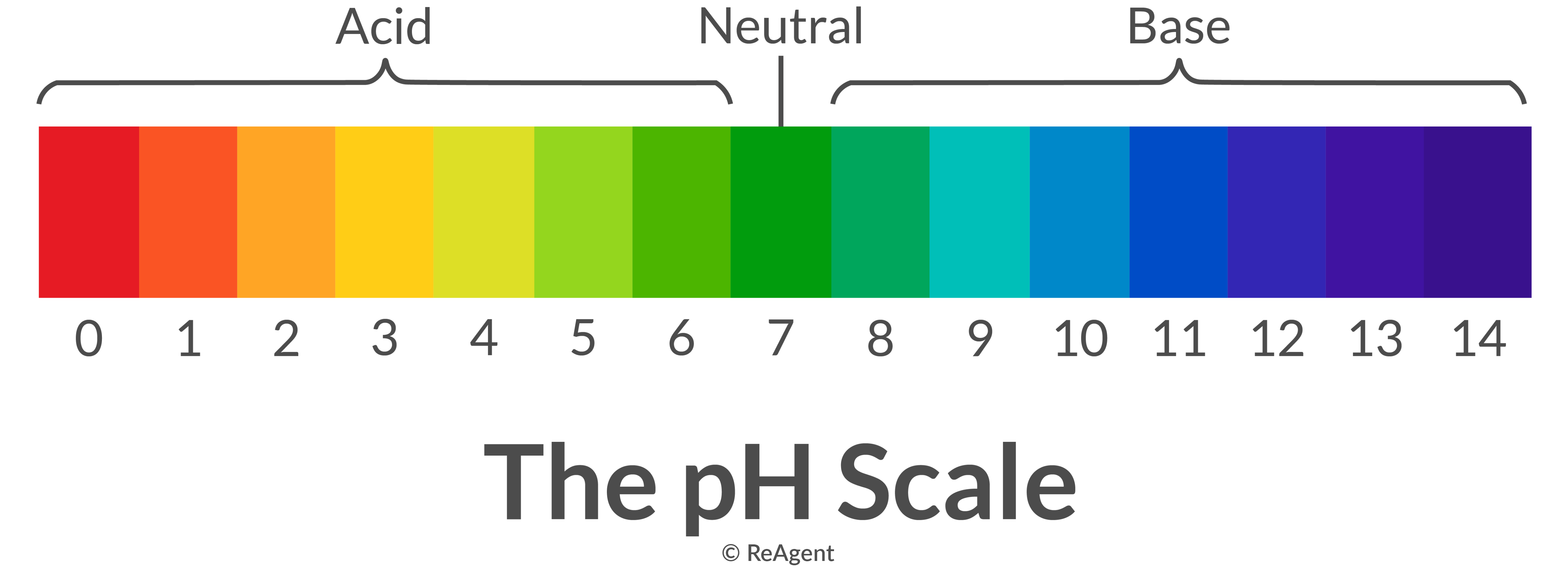
It will start out red and with a pH of about 1. As sodium hydroxide is added, the colour will change from red to orange to yellow to green. Once it is green, its pH has increased to 7 which is neutral (water has been made). H+ + OH- --> H2O
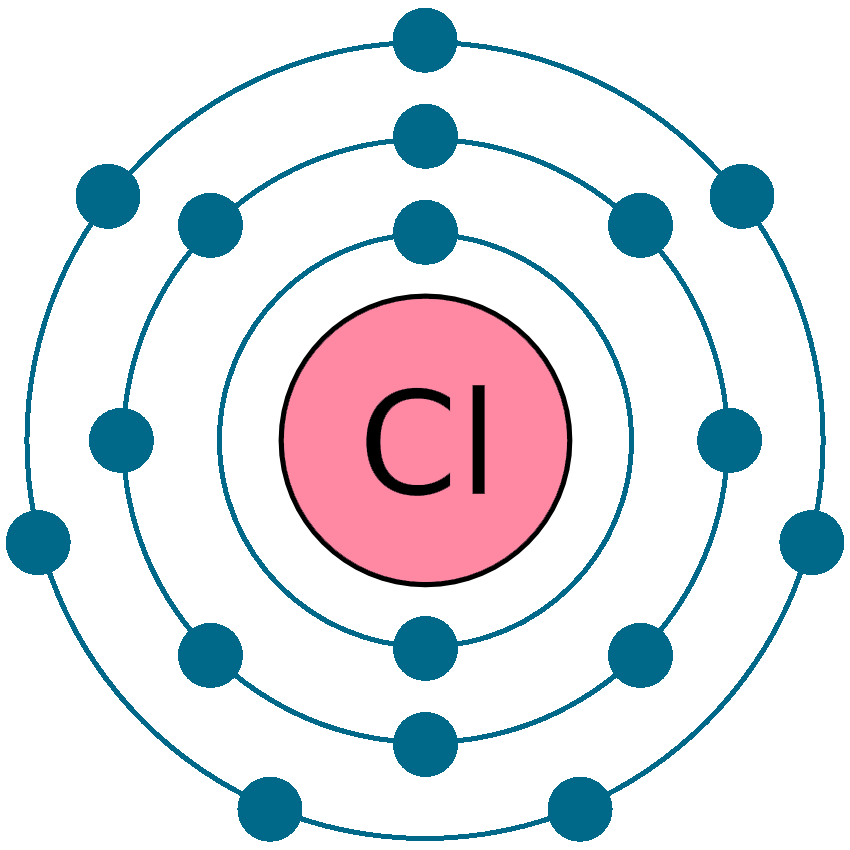
What is the electron arrangement of Chlorine?


2, 8, 7
Name at least 2 qualities about this electrical wire that tells you it must be made of a metal.
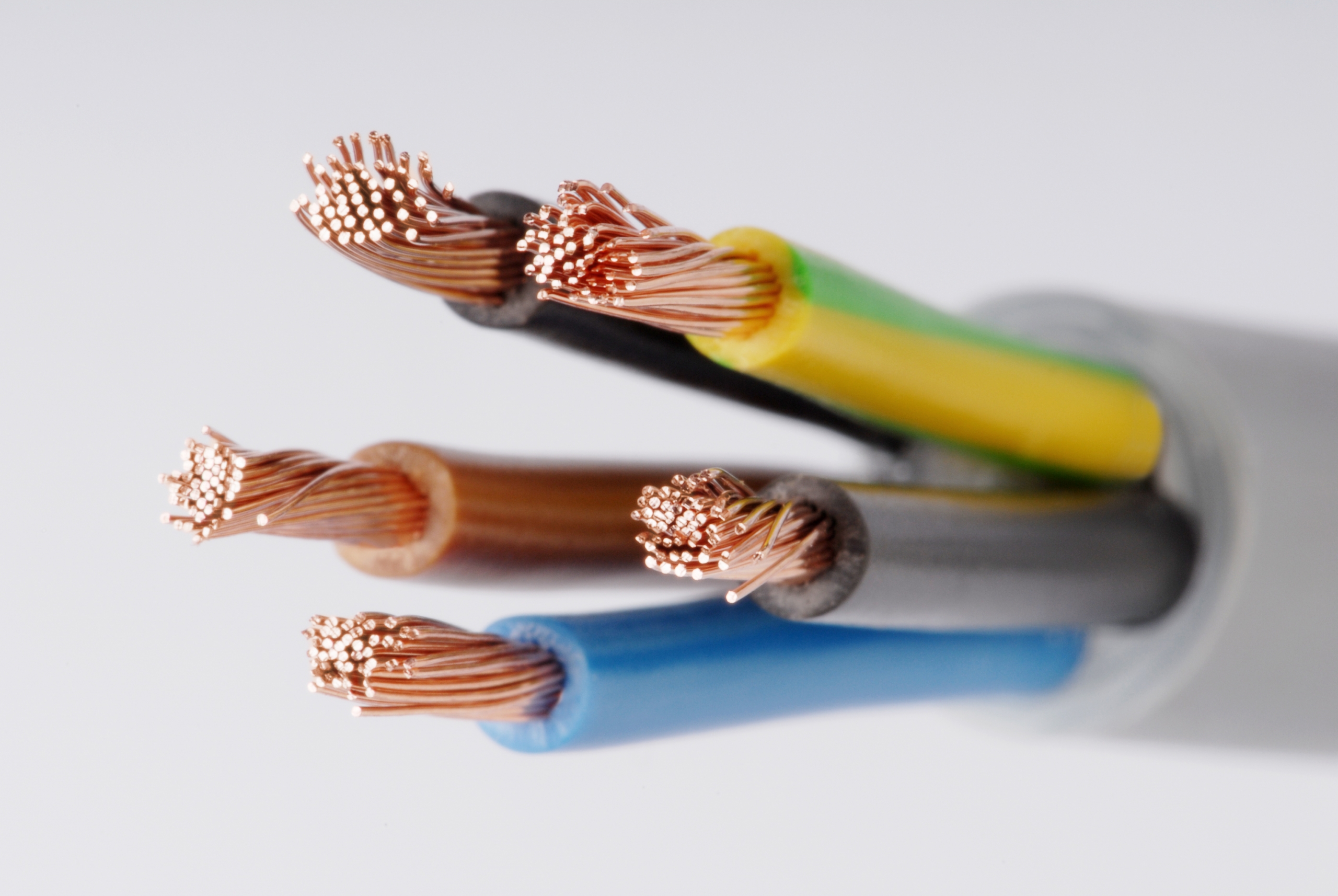
1. It's shiny
2. It can be made into a wire
3. It conducts electricity (lets electricity travel through it)
4. It's solid
Ammonia is basic. What colour would...
1. Phenolphthalein turn when added to ammonia?
2. Litmus paper turn when dipped into ammonia?

1. Phenolphthalein would turn pink
2. Litmus paper would turn (or stay) blue
What are the reactants in a reaction that has the products: salt, water and carbon dioxide?
a) Metal + acid
b) Metal carbonate + acid
c) Metal hydroxide + acid

b) Metal carbonate + acid
A Sulfur ion has a charge of -2. Using its atomic number below, work out how many protons and how many electrons a Sulfur ion has.

16 protons (atomic number = number of protons)
18 electrons (it has gained 2 more electrons, making it negative overall by 2)
Metals want to ______ electrons, this makes them ________
Non-metals want to _______ electrons, this makes them ________
Metals want to lose electrons, this makes them positive
Non-metals want to gain electrons, this makes them negative
Acid rain happens when gases combine with water to make sulfuric and nitric acid, making the rain more acidic than usual. Name 3 issues that this can cause.
- Harms insects and aquatic life
- Damages building and sculptures
- Takes nutrients out of soil that trees and plants need
Explain why atoms in group 1 and 7 (17) are more reactive (quicker to react) than atoms in group 2 and 6 (16)?
Bonus: why are atoms in group 8 (18) not reactive at all?

Because atoms in group 1 and 7 are only one electron away from getting their full outershell to become stable - group 1 needs to lose 1 electron, group 7 needs to gain 1. This makes them very ready to react so they can get their full outershells, compared to those who are further from their full shell.
Group 8 atoms already have full outershells so are stable and don't need to react to lose or gain electrons.