The chemical formula of a water molecule
What is H2O?
The freezing point of pure water?
What is 0°C or 32° Fahrenheit?
Densest:
cold water or warm water
What is cold water?
Salinity
What is salt level?
Lab equipment to measure liquid volume
What is graduated cylinder?
The bond that holds a water molecule together
What is covalent bond?
The freezing point of salt water
What is -1.8°C?
Densest:
pure water or salt water
What is salt water?
________ ppt

What is 17.5 ppt?
Melting
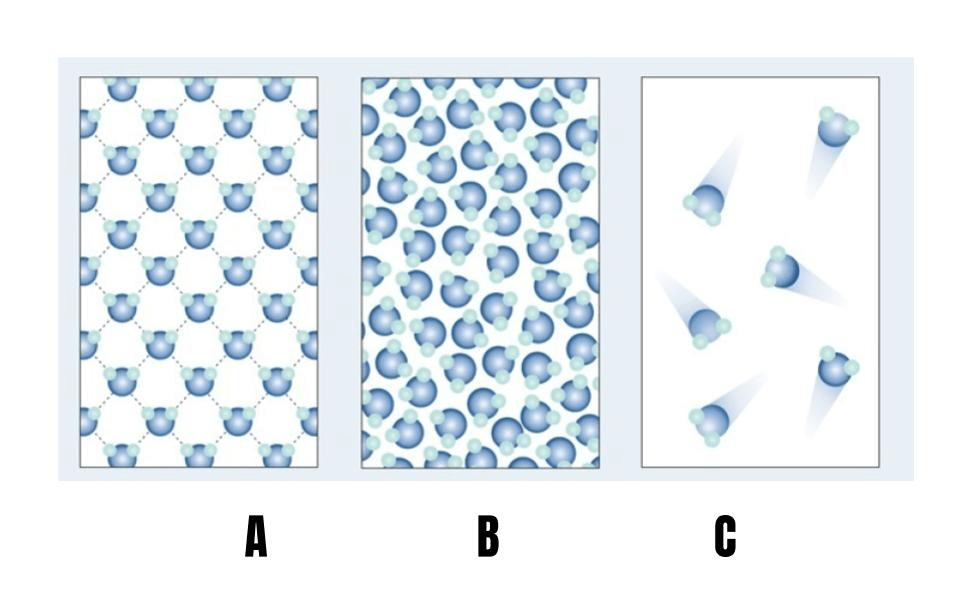
The bond that holds two or more water molecules together
What are hydrogen bonds?
Adhesion
Densest layer of the ocean that is at a constant temperature of 2°C
What is the cold layer?
Measures salinity levels
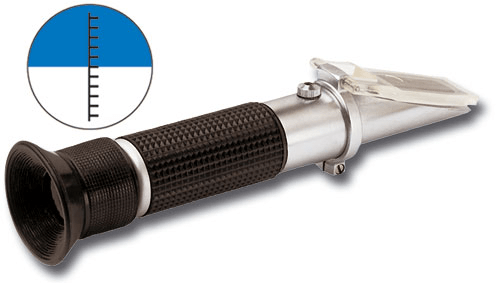
What is a refractometer?
In Fahrenheit, saltwater's freezing point (approximately)
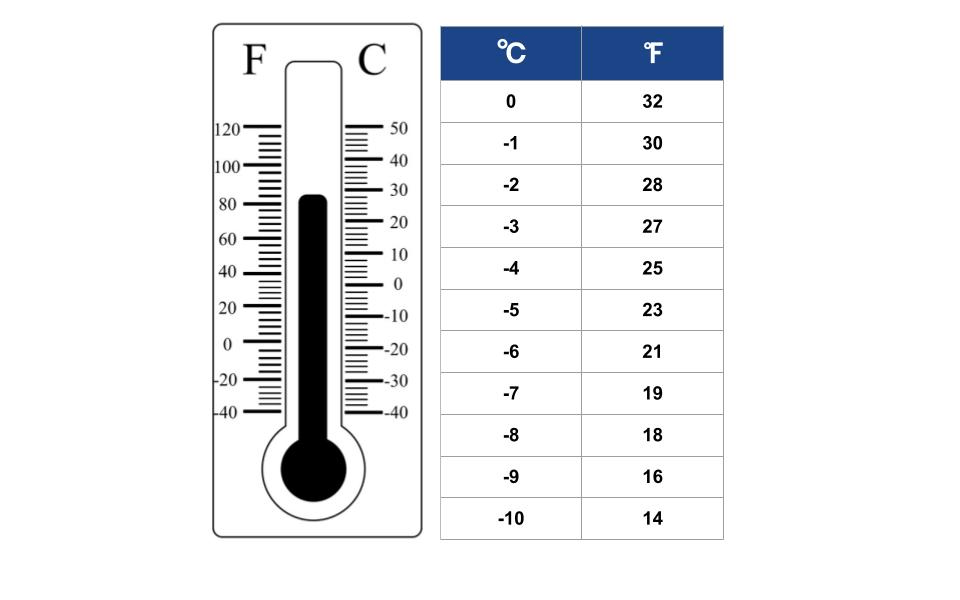
What is 28-29° Fahrenheit?
A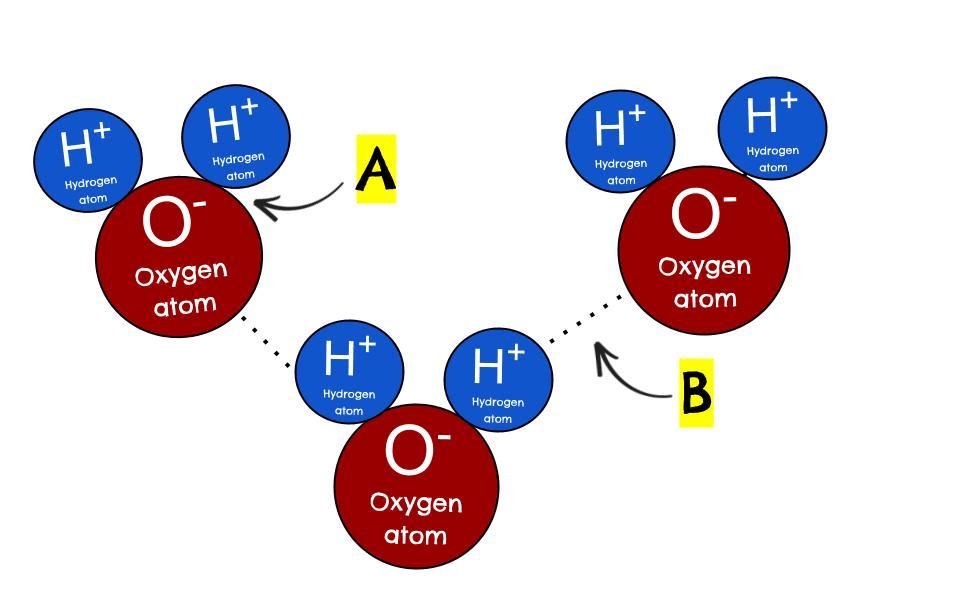
What is a covalent bond?
Water molecules stick together and form a "skin" layer that allow small bugs to walk on the surface of water.
What is surface tension?
Rapid temperature decrease where heat energy is quickly lost
What is thermocline?
Clear water

What is low turbidity?
X-axis
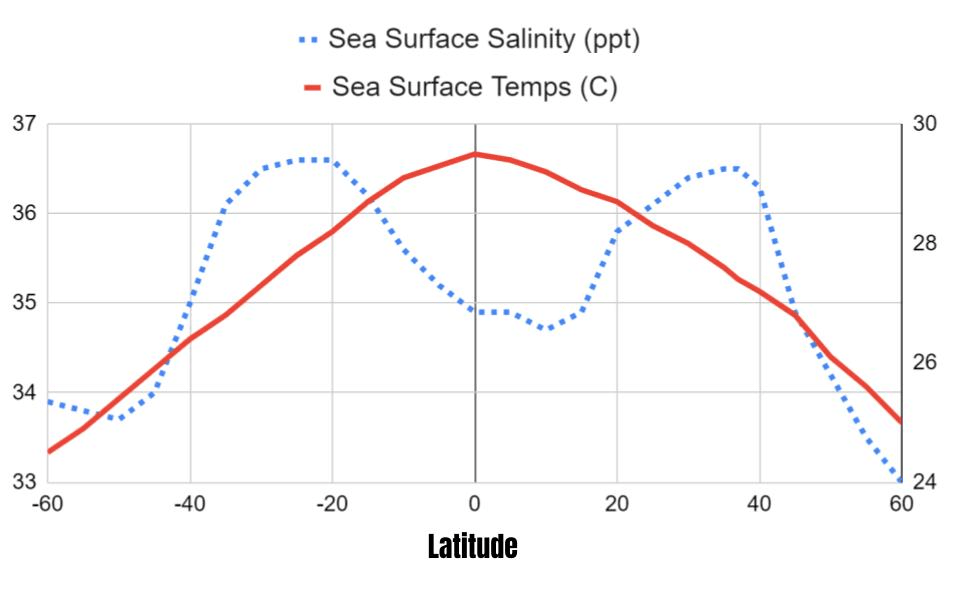
What is latitude?
B
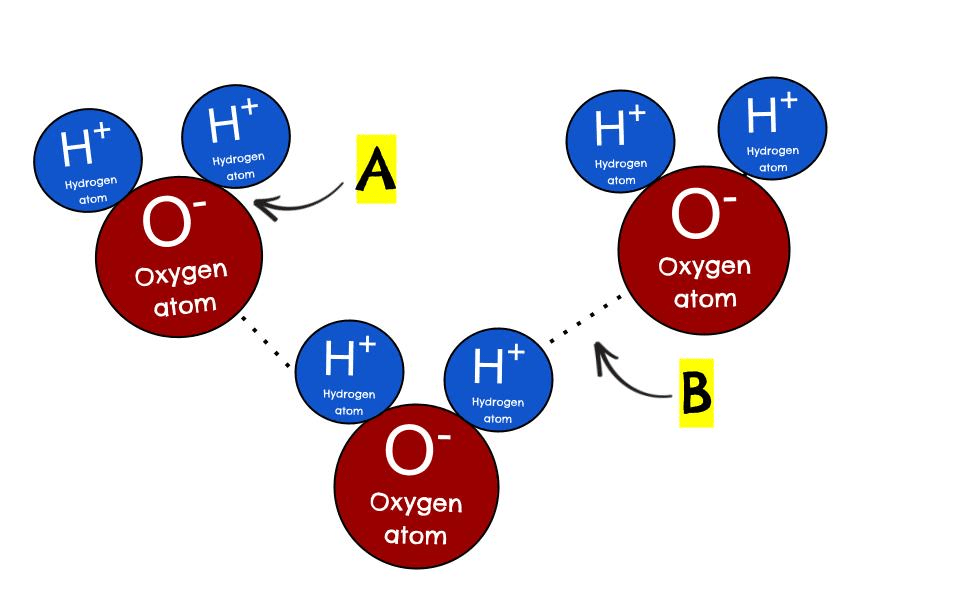
What is a hydrogen bond?
A
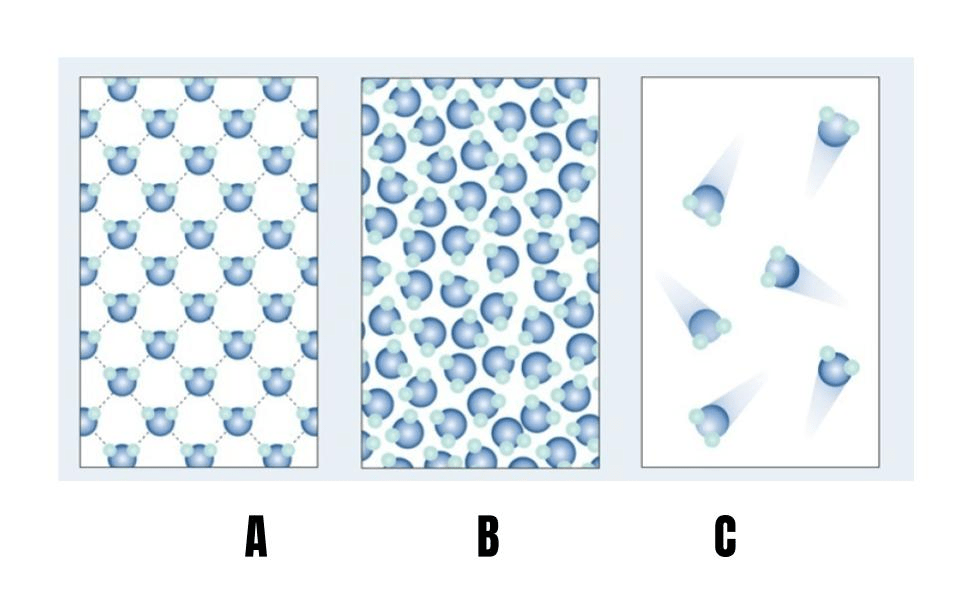
What is a solid?
Densest layer

What is honey?
Highest nutrient level
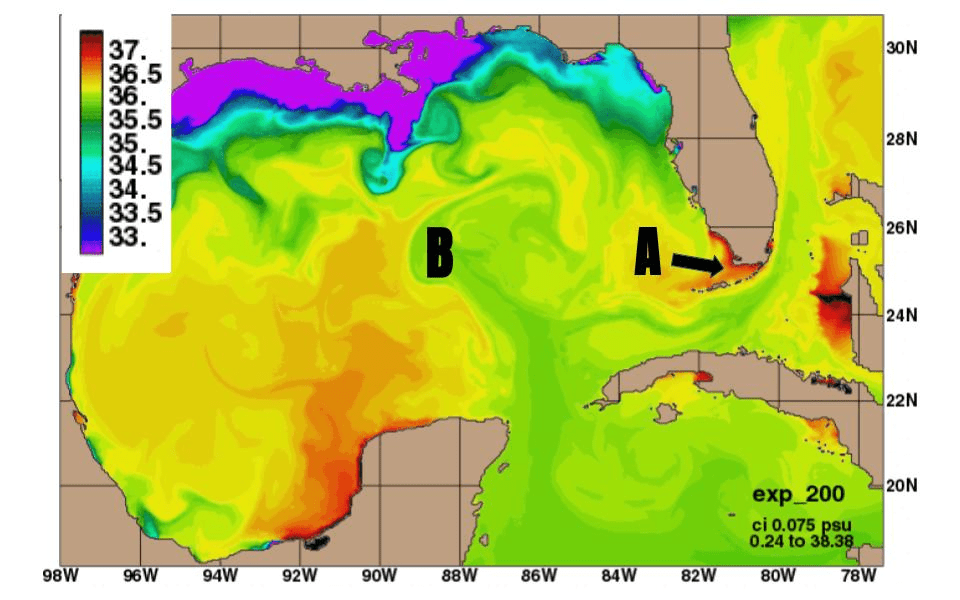
What is A?
Location higher salinity (°S)
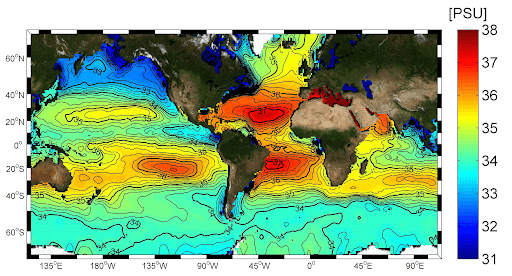
What is 20°S or 20°N?