The force that holds different atoms or ions together into a more stable unit is called a _______ ______
chemical bond
If you have a binary ionic compound whose chemical formula is given, then one of them must be classified as a (metal, nonmetal)____________ and the other must be classified as a (metal, nonmetal) ______________
metal, nonmetal
Metallic bonds form when a metal atom forms a bond with a (metal, nonmetal) ___________ atom.
metal
The anion formed from a phosphorous atom would be called ______ and would have an oxidation number of _______
phosphide, 3-
What kind of chemical bond holds two nonmetal atoms together?
covalent bond (sharing of valence electrons)
What is the bond ratio of metal to nonmetal in this compound? Al2O3
2Al: 3O
Fe(NO3)3
3 Fe atoms, 3 nitrogen atoms, 9 Oxygen atoms
As atoms bond with each other to form a chemical bond, their potential energy (decreases, increases, stays the same) ____.
decreases
Most nonmetal atoms have 4 or more valence electrons, so most will form ionic bonds by (gaining, losing, sharing) _______ valance electrons to form (covalent bonds, negatively charged anions, positively charged cations) ___________________.
gaining, negatively charged anions
What describes the way in which valence electrons are effected by formation of a metallic bond?
They are transferred from one atom to the other; they are shared between the two bonded atoms; they join a sea of electrons that all atoms in the substance share
they join a sea of electrons that all atoms in the substance share
The name of this covalent compound, tetra carbon pentahydride tells you that it contains _____________
4 C atoms and 5 H atoms
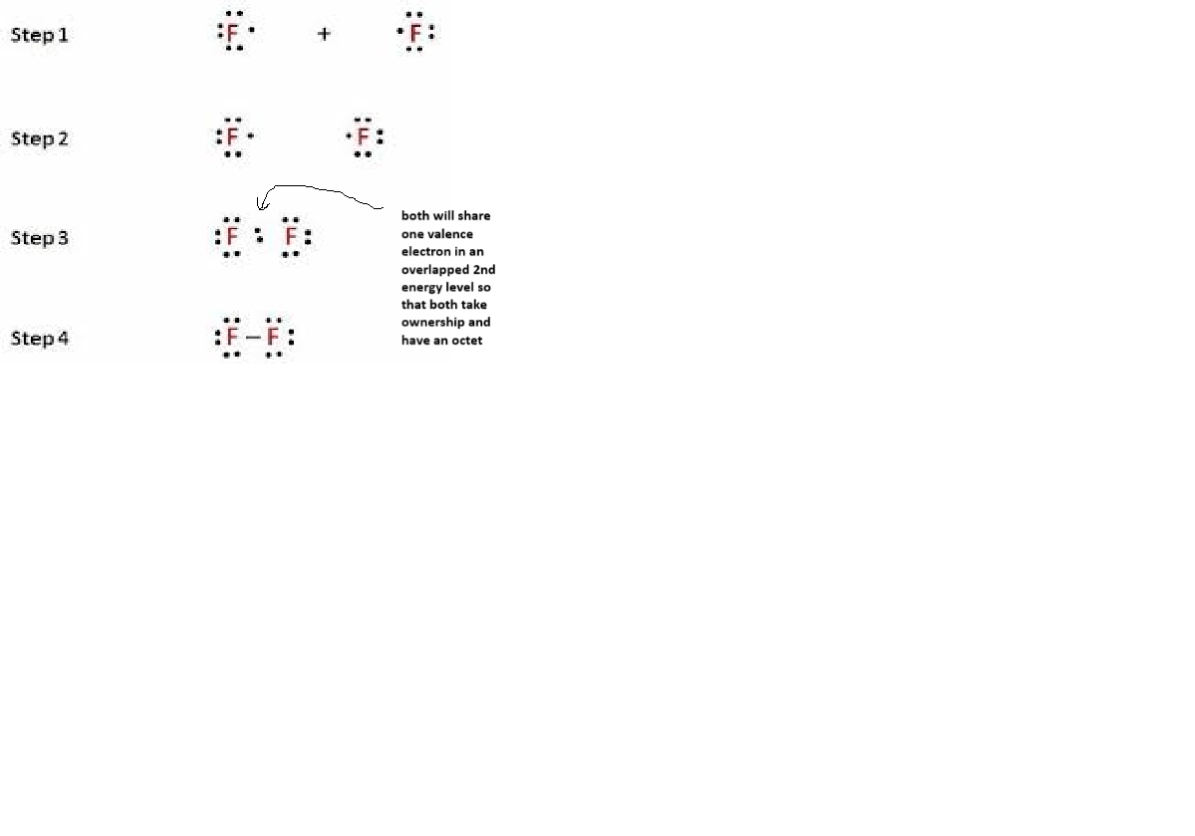
Draw a pair of Lewis dot structures that would show how 2 atoms of Fluorine use covalent bonding to fill the octet rule to become more stable. F2 is a stable diatomic molecule.
what is the bond ratio between compounds formed between alkali metals and halogens? Why?
1 metal: 1 nonmetal
why? alkali metals lose 1 valence electron and halogens take one valence electron
What is the name of this compound
W(PO4)2
tungsten (VI) phosphate (because 3- x 2 is 6-, so the tungsten had oxidation number 6+
Except for the noble gases, which have a valence octet, unreacted atoms are (equal in stability, lower in stability, higher in stability) ________ compared to chemically bonded atoms.
lower in stability
Most metal atoms have 4 or fewer valence electrons, so most will _______ valence electrons to form ions that have a ___________ charge.
lose, positive
List three properties of all metallically bonded substances
good conductors, high malleability, high ductility
Which of these correctly describes Sc2O3 ?
2 Sc3- ions and 3 O2+ ions
2 Sc3+ ions and 3 O2- ions
2 Sc2+ ions and 3 O3- ions
2 Sc2- ions and 3 O3+ ions
2 Sc3+ ions and 3 O2- ions
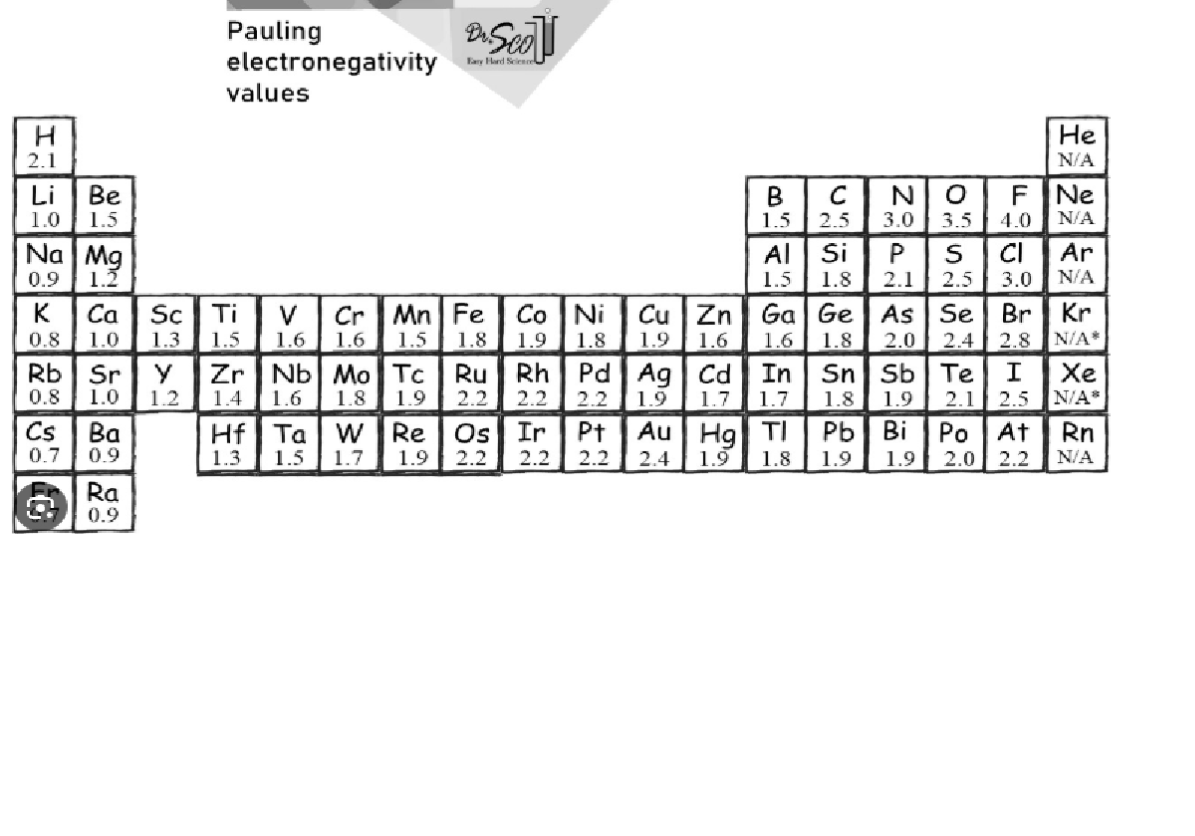 Use electronegativity values to classify each of these bonds as
Use electronegativity values to classify each of these bonds as
nonpolar covalent (0-0.4)
polar covalent (>0.4 but < 1.7)
ionic (>1.7)
Cs to F C to H C to O
Cs to F 4.0-0.7 = 3.3 ionic
C to H 2.5-2.1 = 0.4 nonpolar covalent
C to O 3.5 - 2.5 = 1.0 polar covalent
So Cs transfers its valence electron to F
So C and H share electrons nearly equally
So C and O share unequally with the shared electrons nearer O most of the time.
what is the bond ratio of any member of alkali earth metal with any halogen? why?
2 metals: 1 nonmetal
why? the metals lose 2 valence electrons, and the nonmetals take one
write the chemical formula of ammonium cyanide
All atoms are lower in potential energy and are more stable when they have obeyed the _________ rule and have a filled valence shell; EXCEPT for the _________ _______, this means that atoms in chemical compounds are more stable than unreacted atoms!
octet, noble gases
The sum of all charges in an ionically bonded compound is equal to ____________.
zero
Which of these pairs of atoms would be bonded by a metallic bond?
Zn and Cu or Zn and O or O and N
or all of these
Zn and Cu
What is the oxidation of an alkali earth metal in most compounds, and why?
+2 because these metal atoms lose their 2 valence electrons to become as stable as the previous noble gas
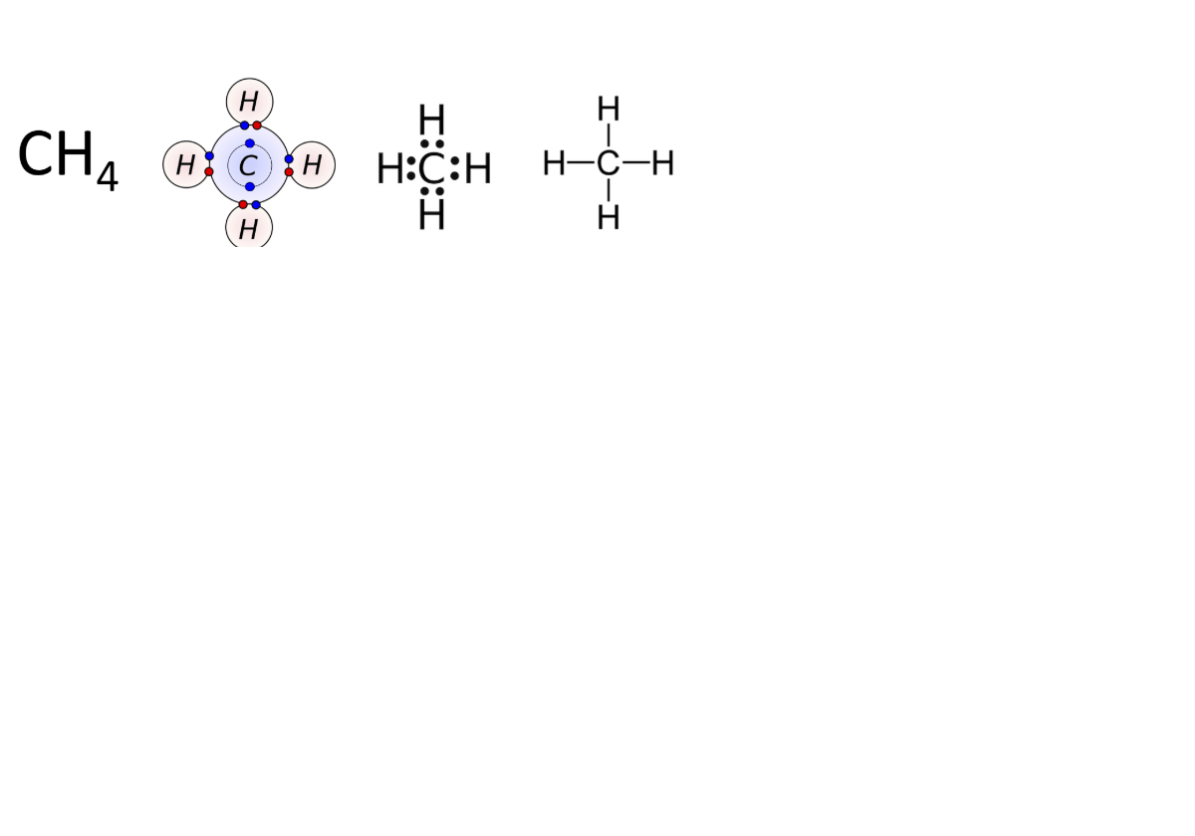
What is the total number of Lewis dots ( or lines for single covalent bonds -) that will be in contact with each H in a correctly drawn covalent compound's Lewis dot structure?
What about the total number Lewis dots (or single covalent bonds) for every ogther nonmetal in a correctly drawn molecule's Lewis strucutre?
for H: two dots which equal to one single bond dash : or __ example H:H or H__H
all other nonmetals: 8 dots in 4 pairs or 4 lines
why? the metals lose 3 valence electrons, while the nonmetals take only 2, so the ratio of 3 to 2 allows all the donated valence electrons to be accepted by nonmetals
(NH4)2CO3
ionic because ammonium is a positively charged polyatomic ion (so this ionic compound can have a cation without having any metal atoms participating in it)
ammonium carbonate
The three kinds of chemical bonds used by non-noble gas atoms as a way to meet the octet rule are: _____ bonds, ___________ bonds, and _________ bonds. Put them in alphabetical order for scoring.
covalent, ionic, metallic
Which of these pairs of atoms would react to form an ionic bond?
Mg and Mg or Mg and O or
O and N or all of these pairs
Mg and O
Homogeneous mixtures of metal elements that are metallically bonded (like 10K or 14K gold, brass, or steel) are called ____.
alloys
On many periodic tables, the most common oxidation number of a transition metal is shown in bold print. What is the most common oxidation number for Tungsten, W, atomic number 74?
6+
Seven of the elements exist as diatomic (2 covalently bonded atoms of the same element) molecules: H2, N2, O2, F2, Cl2, Br2, I2. How do you know that the bonds in each of these diatomic molecules is covalent, not either ionic or metallic?
because both atoms in the molecule's chemical bond are nonmetals ( O nonmetal ot O nonmetal or N nonmetal to N nonmetal etc) and covalent bonds from between two nonmetal atoms since both have relatively high electronegativity.
what is the bond ratio of tricarbon octahydride?
3C to 8 H
What is a polyatomic ion?
a group of covalently bonded atoms that carry a charge because they have either taken or lost valence electrons to become stable
Metals lose valence electrons to become as stable as the (next, previous) ______noble gas in the periodic table, whereas nonmetals gain valence electrons to become as stable as the (next, previous) ______noble gas in the periodic table.
previous for metals, next for nonmetals
When silver (I) combines with oxygen to form silver (I) oxide, the charge of the silver ion is: _______
+1
How are all of the properties of metallic substances related to the nature of metallic bonds?
metallic bonds are made comprised of a "sea of free electrons" that are equally shared by all metal cations in an entire piece of the metal--so the electrons flow freely to allow shape changes and to allow electricity/heat to flow easily
If transition metal Osmium forms a compound called Osmium (IV) tetraiodide, then what is the charge of the osmium ion?
4+
In a correctly drawn Lewis dot structure for a stable covalent compound (molecule), every nonmetal atom EXCEPT H, will be surrounded by a total of _____ dots or ____ lines (single covalent bonds made of a pair of shared valenced electrons).
8 dots or 4 lines (the Lewis dots may all be in shared pairs that are involved in a covalent bond OR they may be combined in covalent bonds as well as be unshared pairs of valence electrons)
use lewis dot structures to show the bonding ratio between a group 13 metal and a group 16 nonmetal.

Write the chemical formula for vanadium (IV) phosphate, then list the number of each atom present in one formula unit.
vanadium is #23
V3(PO4)4
3V atoms, 4 P atoms, 16 O atoms
To draw the lewis dot structure for the elements that will bond to form a compound, then you must know the number of _______________ that each atom contains.
valence electron, because the dots in a Lewis dot structure are the valence electrons
What is the oxidation number of iron (symbol Fe) in the compound FeF3?
3+
Why are ionically and covalently bonded substances poor conductors and brittle instead of either malleable or ductile?
valence electrons in ionic and covalent substances are NOT free to move around
If a metal (X) with 2 lewis dots were to react with a nonmetal (Y) with 5 lewis dots, then what is the chemical formula (XnYn) that will form?
X3Y2 for example
metal w/ 2 lewis dots could be an alkali earth metal and nonmetal with 5 dots with be in group 15 ...like Ca reacting with N
Ca2+N3- forms Ca3N2
Which of these shows one correctly drawn Lewis structure for a covalent molecule? (because one or more atoms does not have 2 dots on H or does not have 8 dots for all other atoms)
the only correct drawing is d--rest are incorrect: a is incorrect due to unpaired electrons (7 total for the rightmost C and c is incorrect due to 9 electrons on the leftmost C and 3 on the leftmost H, b is incorrect due to the leftmost C only having 6 electrons, as well as the middle C.
If there is a metal having 2 valence electrons, and a nonmetal having 4 valence electrons, then which of these chemical formulas would have the correct bond ratio? Use X for metal. Use Y for nonmetal
XY2 because the metal will lose both valence electrons (its a member of group 2 with 2 valence electrons) and the nonmetal will gain 4 valence electrons (its a member of group 14 with 4 valence electrons)
initial criss cross result X4Y2 simplified to X2Y
explain why vanadium (IV) phosphate V3(PO4)4 is neutral
3 X 4+ = 12+
4 X 3- = 12-
sum of these charges is zero