The charge of Oxygen when it gains two electrons
negative 2
This element has the greatest electronegativity.
Fluorine
45 in Nickel-45 stands for
Avg Atomic Mass
The weight of a proton
1 amu
Number of protons and neutrons
atomic mass
The main subatomic particles in the nucleus of an atom.
Protons and Neutrons
Between Calcium and Arsenic this has the greatest atomic radius
Calcium
The Avg. Atomic Mass for 99% Hydrogen-1, 0.8% Hydrogen-2, 0.2% Hydrogen-3
1.012 amu
Electrons carry the majority of the atomic weight in an atom
T or F?
False
Total Charge of an Atom's Nucleus
Nuclear Charge
The number of protons P-3
18
Chemical families have the same ______.
Properites
Average Atomic Mass of:
76% chlorine-35
24% chlorine-37
35.48 amu
Bohr Model of Phosphorous
The amount of energy to remove an electron from an atom
ionization energy
A non metal ______ electrons to become a _____ and a metal ______ an electron to become a _______.
A non-metal GAINS electron to become an ANION and a metal LOSES electrons to become a CATION
This element is the most reactive because it has the largest atomic radius.
Francium
Nitrogen’s Avg atomic mass is 14.007. Which isotope is closer to the average.
¹²N or ¹⁸N
Nitrogen-12
What element does this Bohr Model represent?

Ar
Electronegativity
The tendency of an atom to gain an electron
In Al+3 there are ___ electrons
10
Which element has the lowest ionization energy between Ag, Sb, Rb, and I?
Rubidium
Calculate the average atomic mass
Mg-24: mass 23.985 percentage 78.7%
Mg-25: mass 24.986 percentage 10.13%
Mg-26: mass 25.983 percentage 11.17%
24.31 amu
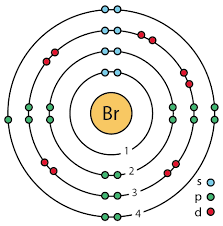
Draw the Bohr model of bromine.
The law that explains the periodic trends by describing that the force is directly proportional to the product of their charges and inversely proportional to the distance.
Coulomb's Law