What 4 elements (full names) are in the molecule below?
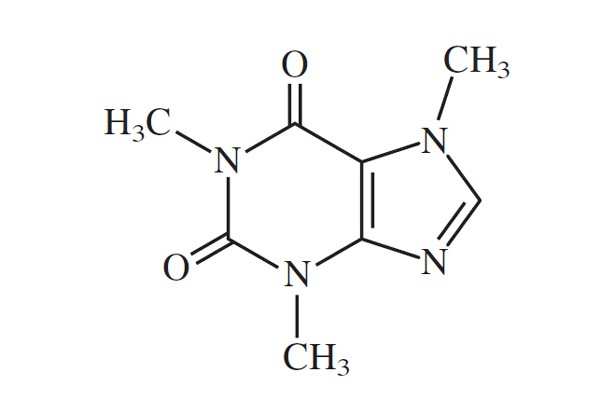
Carbon, Nitrogen, Oxygen, Hydrogen
The smallest particles of an element, which are made up of protons, neutrons, and electrons. They have a neutral charge as the protons equal the number of electrons.
Define the term ion.
A positively or negatively charged particle.
What are 3 signs of a chemical reaction?
Change in colour, temperature, appearance
Gas being produced
Change in pH
H2 + Cl2 -> HCl
H2 + Cl2 -> 2HCl
What are 2 ways we refer to the '22' in titanium?
BONUS QUESTION: What is most likely the most abundant isotope of titanium if its relative atomic mass is 47.867?

Atomic number, proton number
48
By the number of protons.
What is a anion?
Negatively charged ion
How could we tell that the metals we tested were alkaline? Be as specific as possible
Mg + O2 -> MgO
2Mg + O2 -> 2MgO
He, Ne, Ar, Kr, Xe, Rn
Draw the electron configuration (Bohr Model) of Silicon.
![]()
Define the term ionic bond.
The electrostatic attraction between oppositely charge ions.
Why do nobel gases rarely react with other elements?
They are stable due to full outer shells of electrons.
Fe2O3 + Al -> Fe + Al2O3
Fe2O3 + 2Al -> 2Fe + Al2O3
In the periodic table, how do we organise groups atoms with similar properties?
They are in the same vertical column
What is the purpose of a neutron?
They help stabilise the nucleus of an atom.
What is is meant by the term valence electrons?
The electrons in the outermost energy level of an atom or ion.
How do we prevent highly reactive chemicals from mixing with air or water?
Keep it in oil or hexane
Al + HCl -> H2 + AlCl3
2Al + 6HCl -> 3H2 + 2AlCl3
State an element with 5 valence electrons
Nitrogen, phosphorus, arsenic, antimony, and bismuth
What is the relative mass of electrons compared to protons?
BONUS: Why don't we include the mass of electrons when calculating relative atomic mass?
1/2000
Because they are too small and don't make a big difference to the overall mass.
Draw the ionic bonding between Calcium and Chlorine (draw as Lewis structures).

What do we call a reaction which produces a large amount of heat, or even fire?
Exothermic
SO2 + Li2Se -> SSe2 + LiO
SO2 + 2Li2Se -> SSe2 + 2LiO