Who proposed the idea that matter is composed of indivisible particles known as atoms?
John Dalton
What are the three main subatomic particles of an atom?
Protons, neutrons, and electrons
What are isotopes?
Atoms of the same element with different numbers of neutrons.
If an element has 6 protons and 8 neutrons, what is its mass number?
14
What is the charge of an ion that has 10 protons and 8 electrons?
+2 (it is a cation)
What model of the atom did J.J. Thomson create after discovering the electron?
Plum pudding model
Where are protons and neutrons located in an atom?
In the nucleus
The atomic number lets us know what about an element?
The number of protons in an element
If an atom has 6 protons and 7 neutrons, what is its isotope notation?
Carbon-13 (written as 13C)
How do you determine the charge of an ion?
By comparing the number of protons to electrons.
What was Ernest Rutherford's major finding in his gold foil experiment?The nucleus is small and dense, and most of the atom is empty space.
The nucleus is small and dense, and most of the atom is empty space.
What is the charge of an electron?
Negative charge
To solve for mass number you need to?
Add the protons and neutrons
Calculate the number of neutrons in an isotope of sodium with a mass number of 23.
12 neutrons (23 - 11 protons)
If an atom gains an electron, what type of ion does it become?
An anion
Which scientist is known for the planetary model of the atom?
Niels Bohr
What determines the atomic number of an element?
The number of protons in the nucleus
To solve for charge you need to?
Subtract protons from electrons
If an isotope of chlorine has 18 neutrons, what is its mass number?
35 (since chlorine has 17 protons, 17+18=3517+18=35)
What do you call an atom with a different number of protons and electrons?
An ion
What theory did Schrödinger contribute to the understanding of atomic structure?
Quantum mechanical model of the atom
How do you find the mass number of an atom?
By adding the number of protons and neutrons
How does the presence of isotopes affect the average atomic mass of an element?
It is a weighted average based on the relative abundance of each isotope.
What is the Isotopic Notation for this bohr model?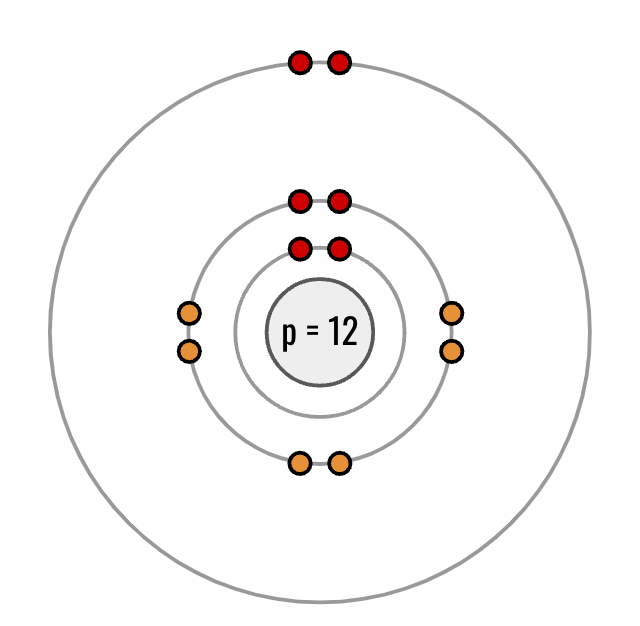
2412Mg
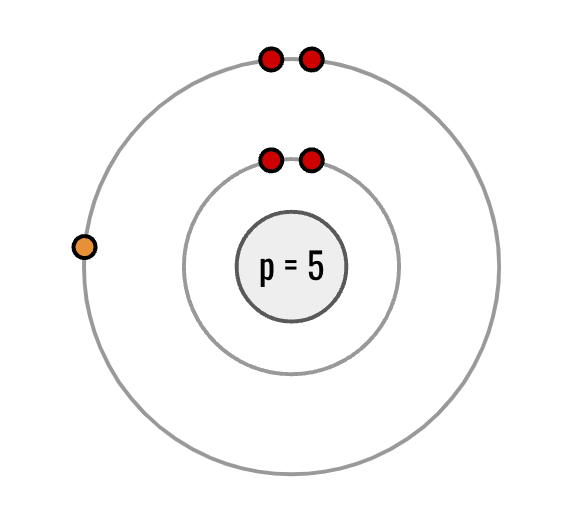
What is the bohr model for Boron?