What is the charge of a proton?
positive
Isotopes of an element have the same number of ___________ but a different number of _______.
Protons neutrons
Which model do scientist shows the electron in rings outside the nueclus?
Bohr
What is the average atomic mass of Lithium?
6.941
the proton
What 2 subatomic particles are found in the nucleus?
Proton and Neutron
How do you calculated the number of neutrons in an element?
Mass Number minus protons = neutrons
Which model is the first to show a dense nucleus?
Rutherford
What's the first thing you should do to find the average atomic mass for this problem?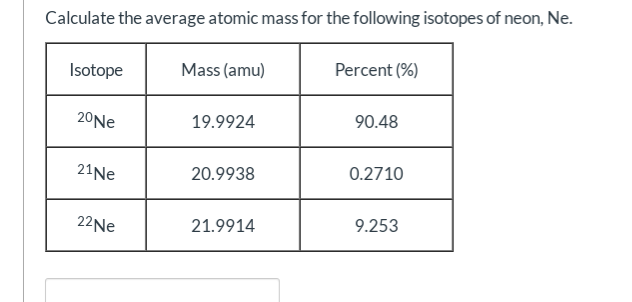
Divide the % by 100
Are these elements the same?
no
Which 2 subatomic particles have the same mass?
Proton and Neutron
How many protons are in this element?
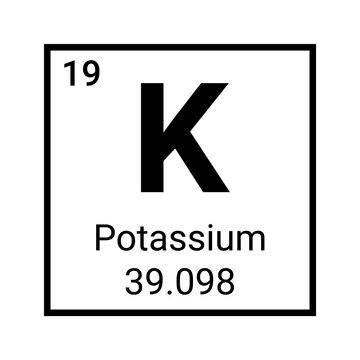
19
Which model shows the electrons in a "cloud"?
Modern/Schrodinger
The average atomic mass for this element should be between what 2 numbers?
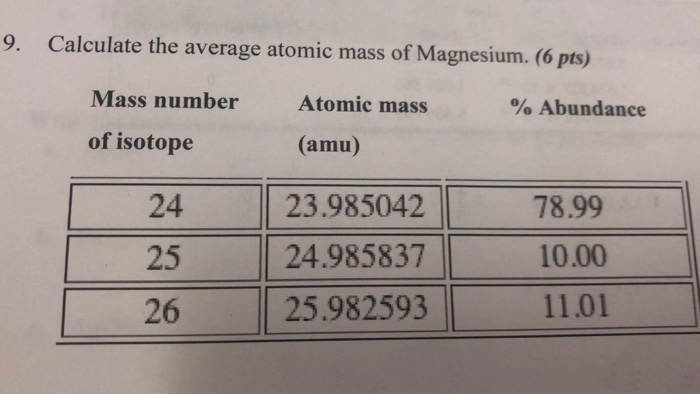
24 and 26
Which of these are the same atom?
Yes
What is the charge of the nucleus of an atom?
Positive
How many neutrons are in Nitrogen-15?
8
Which model shows these merits and limitations?
Merit: Dense nucleus, protons in the nucleus, atom is mostly empty space
Limitations: No neutrons, incorrect movement of electrons
Rutherford
Which isotope would have the greatest affect on the average atomic mass?
J-66
If the pink circle represents the proton, is this still the same element?
No
How many electrons are in this element?
80
How many neutrons are in this element?
8
Why do we mostly use the Bohr model in class?
Its easy to see all the subatomic particles and their location
How is calculating average atomic mass like calculating your overall grade in class
The isotope with the greatest % abundance would be like test grades and have the greater affect on the overall grade.
Lithium has 3 protons. It gains 2 more protons, what element is it now?
Boron