What Charge does an electron have?
As wavelength increases what happens to the frequency?
It decreases
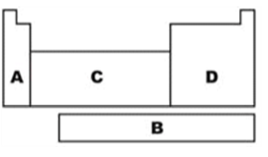 What letter refers to the f-block on the periodic table?
What letter refers to the f-block on the periodic table?
B
Who discovered the nucleus of the atom?
Ernest Rutherford
What is an isotope?
An atom with the same number of protons but a different number of neutrons.
What determines an elements chemcial Properties?
Valence Electrons
What is the formula for electromagentic waves?
C=λv
What group contains the halogen family?
group 17
Who discovered the neutron?
James Chadwick
What is the atomic weight of a proton/neutron?
~1 amu (proton 1.00727 amu, Neutron 1.00865 amu)
What element does the following electron configuration correspond to?
[Ar]4s23d6
Iron(Fe)
what is the wavelength of an x-ray with a frequency of 5.63 x 1019 hz?
C=λv
C= 3.00x108m/s
v=5.63x1019hz
λ=3.00x108/5.63x1019=5.33x10-12m
How many valence electrons does Silicon (Si) have?
4
Who created the plum pudding model?
J.J Thomson
Write the Chemical symbol for an atom with 13 protons and 13 neutrons.
2613Al
What is the electron configuration of chlorine?
1s22s22p63s23p5 or [Ne]3s22p5
A photon of red light has a frequency of 4.7 x 1014hz what is the energy of the photon?
E=hv
h=6.626 x 10-34 Js
c=4.74 x 1014hz
E=6.626x10-34 x 4.74x1014 = 3.14x10-19J
Which diagram depicts the trend of atomic radius?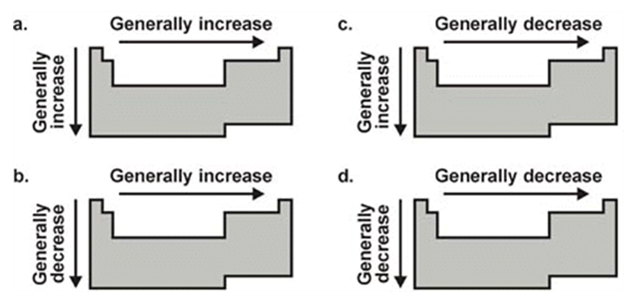
C
Who arranged the first organized periodic table?
Dmitri Mendeleev
how many neutrons does 1737Cl have?
20
Draw the dot structure for oxygen.

what is the wavelength of a photon with an energy of 5.23x10-19?
E=hv
C=λv or v=c/λ
so E=hc/λ
E=5.23x10-19 J, C=3.00x108m/s, h=6.626x10^-34Js
λ = (3.00x108 x 6.626x10-34)/5.25x10-19=3.79 x10-7M
Label all parts in the carbon diagram.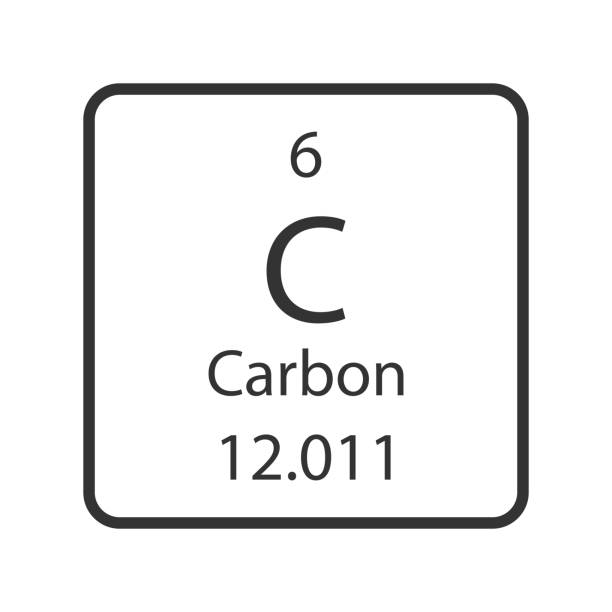
6 = atomic number
C = chemical symbol
Carbon = Chemical name
12.011 = atomic mass
What scientist characterized the law of octaves?
John Newland
An isotope has a mass number of 65 and has 36 neutrons. What element is it?
Copper (Cu)