Make one relevant quantitative observations about this image.
The opossum has 7 babies in all
The opossum has 6 babies on its back and 1 underneath it. 
Name some substances that are not soluble in water.
Examples: oil, rocks, corn starch. 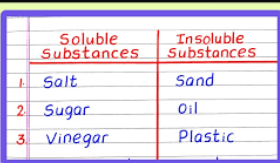
What is a reactant?
Reactants are the starting materials in a chemical reaction,
Name some properties of water.
Liquid at room temperature, clear odorless substance.
Puts out a flame.
Define a chemical reaction
Atoms rearrange to create a new substance
Make Two qualitative observations of this picture.
of this picture.
There is a picture of a bird flying.
The bird has large blue grey wings.
The bird has a long orange beak.
The bird has a long white neck.
Is dissolving sugar in water a physical change or a chemical change? Answer and explain.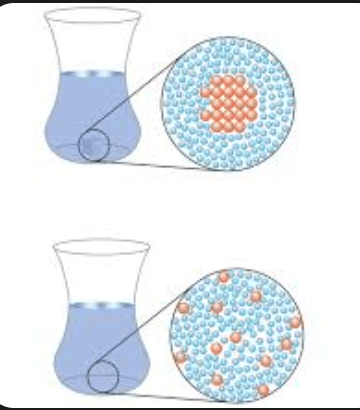
Physical. There is no new substance the particles just get mixed around.
State the law of conservation of matter in one sentence
Double Jeopardy Question
Matter is neither created nor destroyed during a chemical reaction; total atoms remain the same.
Name some properties of carbon dioxide.
Odorless, puts out a flame, invisible, denser then air.
Name signs (evidence) of a chemical reaction?
Bubbling, smoke, a smell, a solid appears, a color change.
What is a relevant observation about this reaction?
The liquid turned orange
A student mixes two clear solutions and the mixture becomes cloudy and gives off a smell. Which observation(s) indicate a chemical reaction and why?
Cloudiness (precipitate) and smell are evidence of chemical change; cloudiness indicates new solid, smell indicates new substances/volatiles.
In a closed system, the total number of atoms before and after a chemical reaction is expected to: increase, decrease, or stay the same? Explain briefly.
Stay the same — atoms are rearranged into new substances but not created or destroyed.
List three properties you could measure to compare a substance before and after a reaction
density, melting point, boiling point, solubility, flammability, odor, color, conductivity
Two substances are mixed and an odor develops this indicates a .............. reactions
chemical
Burning a candle produces light and ash. Is this a physical or chemical change? Explain which new substances form
Burning candle = chemical change; new substances like carbon dioxide and water vapor (and solid soot) form.
Is dissolving a chemical reaction?
Not necessarily. It depends if there are signs of a chemical reaction and if the mixture is new substance cannot be separated and has new properties.
Can the reactants vinegar (acetic acid) and baking soda (sodium bicarbonate) make sodium chloride as a product? Why or why not?
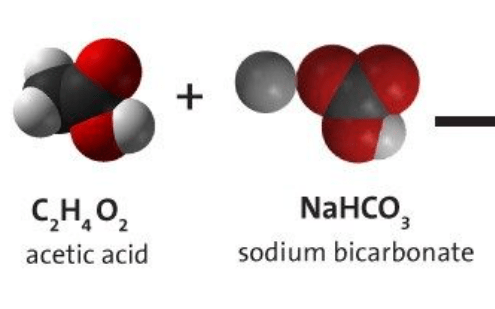
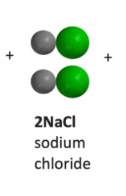
No because the reactants do not have the green chlorine circles and atoms cannot be created or destroyed in a reactions.
Putting a match in a flask titled sideways after a bubbling reaction checks which property of the unknown gas. 
flammability
Adding electric energy to water creates bubbles. How do we know this is a chemical reaction? 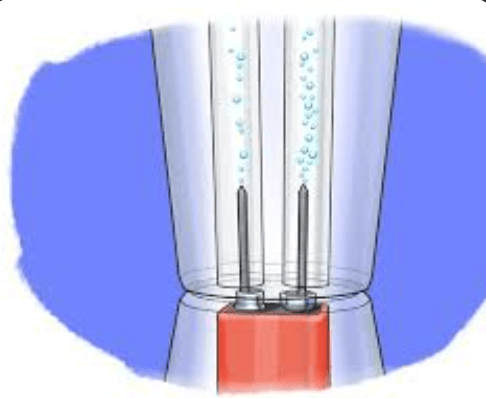
The gas that forms stays a gas at room temperature.
the gas that forms is flammable and creates a popping sound with a match.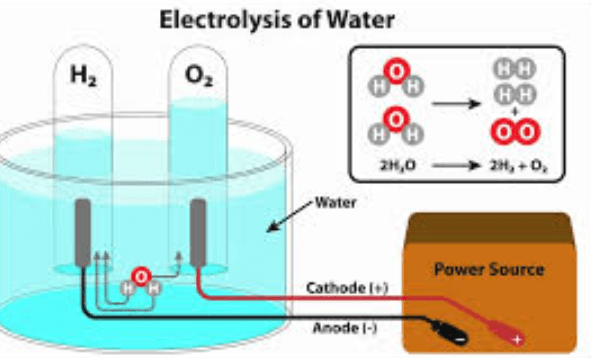
Double Jeopardy Question
What are the products of these reactants?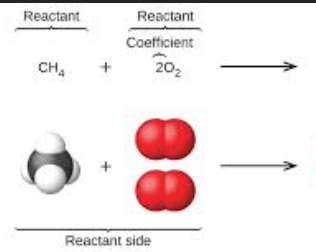
Carbon dioxide and water 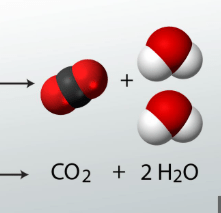
Can something be soluble in water but not soluble in alcohol. Answer and give an example.
Yes Sodium citrate is soluble in water but not in alcohol
Can the reactants vinegar (acetic acid) and baking soda (sodium bicarbonate) make sodium acetate as a product? Why or why not?
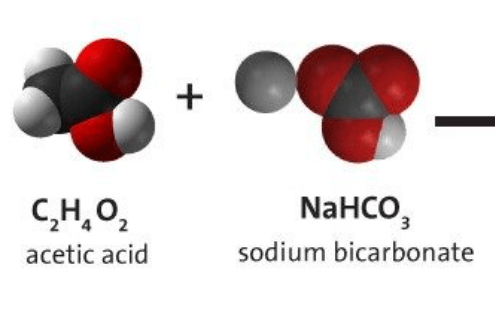
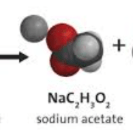
Yes because it has all the atoms available. Sodium, carbon, hydrogen, and oxygen
If we already know a gas is not flammable, it puts out a flame, when the flask is sideways. What test is being shown here. Explain.
It's density test. To see if the not flammable gas is lighter than air. 
Adding heat to water created bubbling, the bubbles popped and were full of a gas. Was this a chemical reaction and how do you know? 
This is not a chemical reaction.
The gas turned back to a liquid at room temperature and the density was the same as water. The gas put a flame out.
Can calcium chloride and sodium bicarbonate make calcium carbonate?Reactants: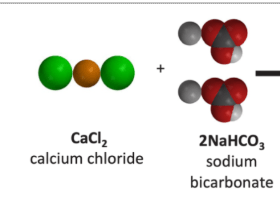 Possible Products:
Possible Products: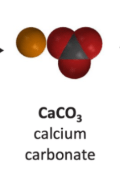
Yes because all the atoms are available to form this product.