Name the 3 states of matter.
Solid, Liquid, Gas
How tightly packed matter is.
Density
How many subatomic particles make up atoms? Name those particles.
3: protons, neutrons, electrons
Elements are arranged by their properties.
What does the prefix "sub" mean?
ex. Subatomic or Submarine
The study of nonliving things and the natural world, focusing on matter.
Physical science

Which state of matter is this?
Solid
The amount of space an object/substance takes up.
Volume
Protons have a _________ charge.
Positive (+)
What are the 3 major categories that elements can fall into?
Metals, Nonmetals, Metalloids
Is water an element? Explain your answer.
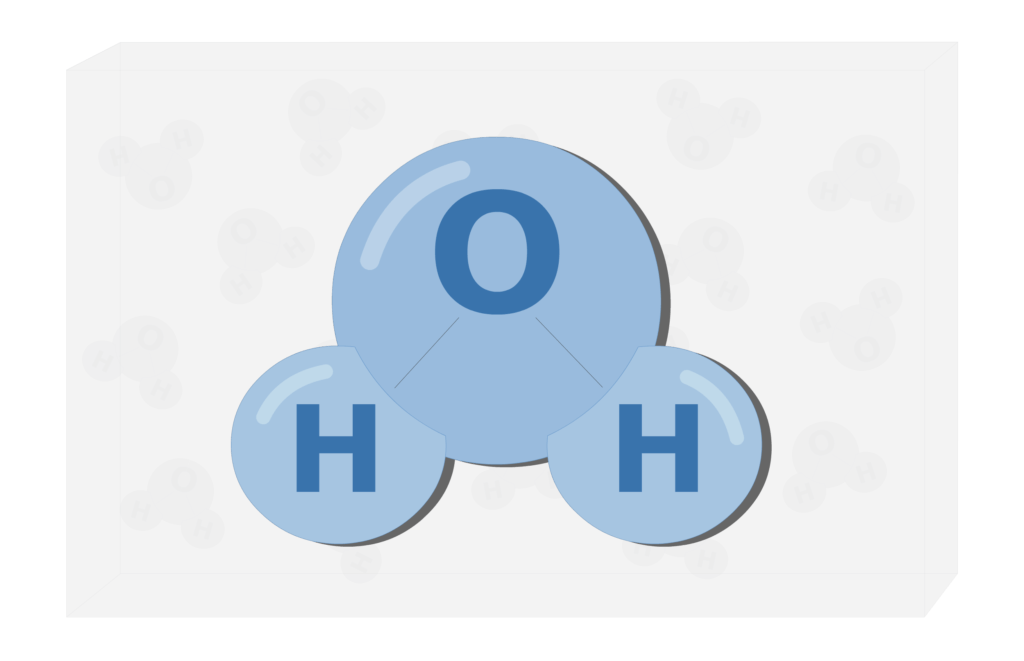
No! It can be broken down into simpler substances (Hydrogen and Oxygen).
Physical properties
What state of matter is this?
Liquid
is flammability a physical or chemical property?
Chemical. In order to observe this property, you must burn the object.
Neutrons have _______ charge.
Neutrons have no charge. They are neutral.
What properties would you expect a metal to display?
Malleability, Luster, Conductivity
What two properties make copper a great metal for making electrical wires?
Malleability and Conductivity.
We are able to shape copper into metal wires that carry an electrical charge.
Properties that can only be measured or observed when a substance changes into a new substance.
Chemical properties

What state of matter is this?
Is reactivity a physical or chemical property?
Chemical
Which particles are found inside the nucleus of an atom?
Protons and Neutrons
What properties would you expect a Nonmetal to display?
Dullness, Brittle, not good conductors (insulator)
Block B
The building blocks of matter
Atoms
How many states of matter have a set volume?
2: Solids and Liquids
Where can you find electrons?
Orbiting the nucleus of an atom
What properties would you expect a metalloid to show?
Properties of both metals and nonmetals.
What is the name of the only metal that is liquid at room temperature?
Mercury
A substance that cannot be broken down into a simpler substance.
Element
How many states of matter have a set shape?
1: Solids
The pH scale ranges from 0-14 :
The closer to 0 you get, the more _______ .
The closer to 14 you get, the more _________ .
7 on the scale represent a ___________ substance.
closer to 0-6 = Acidic
closer to 8-14 = Basic
7 = Neutral
The number of Protons is always equal to the number of _________ .
Electrons

Identify the name and symbol of this element.
Name- Helium
Symbol- He
Where would pure water fall on the pH scal?
7- it's neutral
Where all known elements are displayed. They are arranged by their properties.
Periodic Table
List the states of matter in order from Most dense to Least dense.
Solids, liquids, Gases
What is the difference in an object's hardness and an object malleability?
Hardness= how easily an object dents/scratches
Malleability= the ability of an object to be bent or shaped into a new form without breaking.
An elements atomic number tells us how many __________ an element has.
Protons

Identify the atomic mass and atomic number.
Atomic number- 2
Atomic mass - 4.002602
Who was the first person to propose that all matter was made up of atoms?
Democritus
How acidic or alkaline a substance is.
Describe the movement of gas particles.
They move in all directions to fill their container.
Is a phase change a chemical or physical property?
Physical.
Ex. when ice melts to form liquid water, it's still just water. Boiling water would be the same.
How do we calculate the number of neutrons in an element?
Atomic mass - Atomic number = Neutrons
12.011 - 6 = 6.011

Identify which color represent which group of elements: Metalloids, Nonmetals, Metals
How many elements are on the Periodic table of elements?
118
Objects/substances that carry heat and electricity well.
Conductors