This substance is known as one of the most "difficult" and "uncooperative" liquids due to its high specific heat value of 4.18 J/gC
water
In electrochemistry, what is the word to describe electrons moving down a wire?
electricity
The ability to supply heat or do work
Energy
The SI unit of heat, work, and energy is this.
Joule
the amount of energy required to get chemical reactions to proceed
activation energy
What does the "delta T" mean?
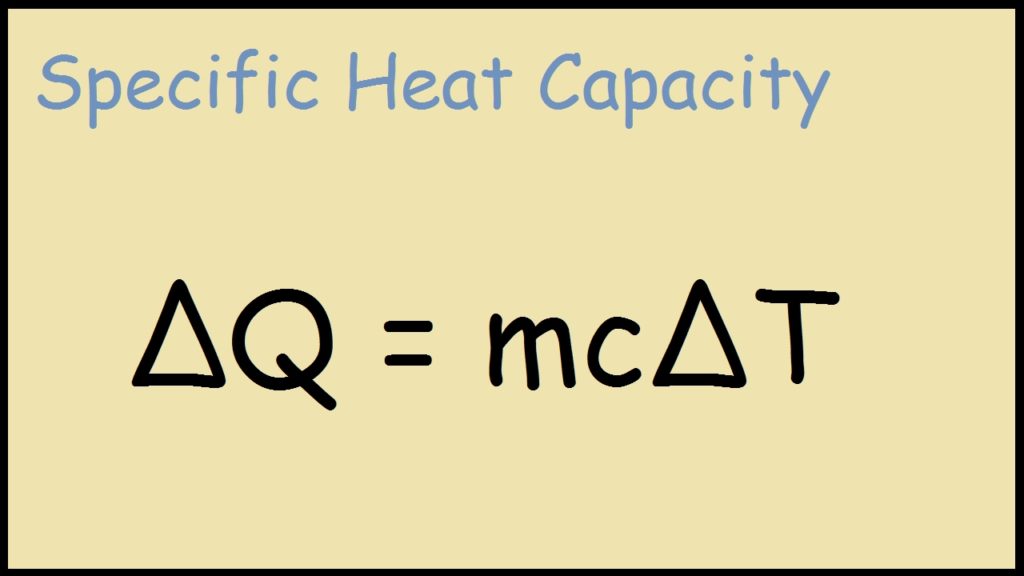
delta T is change in temperature
In electrochemistry, electrons will hop from one metal to another metal in a reduction/oxidation reaction. What is the shortened form of this?
redox reaction
A reaction that releases heat is called...
Exothermic
a reaction that absorbs energy instead of releasing it
Endothermic
this word refers to how much of a substance is dissolved into another substance
concentration
Explain what a reversible reaction is.

A chemical reactions which can proceed in the forward and backward direction
What is the most reactive metal on the list?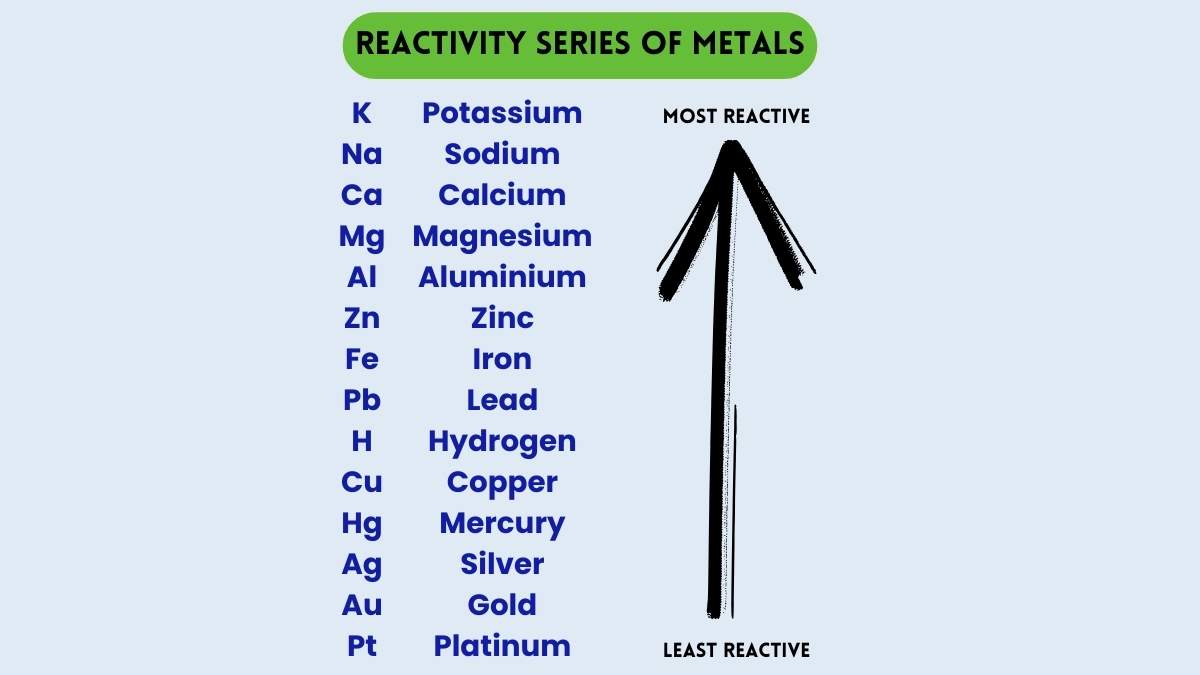
potassium
The quantity of heat required to raise the temperature of 1 gram of a substance by 1 degree C.
specific heat OR specific heat capacity
___________thermic reaction
exothermic
Crushing up large particles into much smaller particles speeds up reactions by increasing the __________ area
surface area
In a battery, the redox reaction continues until it reaches equilibrium. What is the voltage produced by the battery at this point?
zero, you have a dead battery
A battery must have a chemical solution that conducts electricity. This type of solution is called an:
electrolyte
The temperature of this room right now in Celsius (accept answers within 10 degrees)
roughly 68 degrees Fahrenheit or 20 degrees Celsius
name three temperature scales used by:
1. Americans
2. citizens worldwide
3. mostly scientists
Fahrenheit, Celsius, Kelvin
this theory explains reaction rates as how likely particles are to bump into each in the correct orientation
collision theory
When a reversible reaction reaches equilibrium, which is greater? Rate of reactants forming or rate of products forming?
At equilibrium they are equal
These devices are called
voltmeters / multimeters
A lab technique used to measure the amount of heat transferred to or from a substance. (Can simply use a styrofoam cup and a thermometer)
Calorimetry
which has more energy?
A piece of gum with 4.184 Joules of energy or a raisin with 1 calorie of energy?
equal, they are equivalent
4.184 J = 1 cal
a substance that helps to increase the rate of a chemical reaction without actually being involved in the reaction
catalyst
