What is the formula for density?
D=M/V

Quartz,Topaz and Gypsum, Diamond.
List these minerals from hardest to softest
Diamond,Topaz,Quartz,Gypsum
The vertical columns on the periodic table?
Groups
Is this potential or kinetic energy?
Potential energy
What element has an atomic mass 107.87?
Silver
An irregularly shaped piece of gold was lowered into a graduated cylinder holding a volume of water equal to 17 ml. The height of the water rose to 20 ml. If the mass of the gold was 27 g, what was its density?
9g/ml 3
Are Minerals organic or inorganic?
Inorganic
Which element is a metalloid? Silicon, Boron, Oxygen ,Aluminum, Sulfur
Boron and Silicon
In the lab, a student wants to identify two different minerals. So, she takes them and scrapes them across a plate to see the color of the powder they leave behind. What property of minerals is she testing?
Streak
A comic book has a mass of 5g and volume of 100 mL. What is the density?
0.05g/ml 3
A tv has a mass of 4g and density of 88gm/L. What is the volume?
0.045ml
What is the inorganic ?
A material that has never been alive
A ball is rolling down a hill. What kind of energy does it have.
It is losing potential energy and gaining kinetic energy.
The splitting of a mineral along smooth, flat surfaces
cleavage
How well a substance can be hammered into sheets.
Malleability
Which item has more PE a watermelon weighing 25kg or a mango that weighs 14kg
The watermelon because it has more weight
When a metal can be made into thin wires, we say it is
Ductile


Where do you find the metals and nonmetals
Metals to the left side and non-metals to the right side
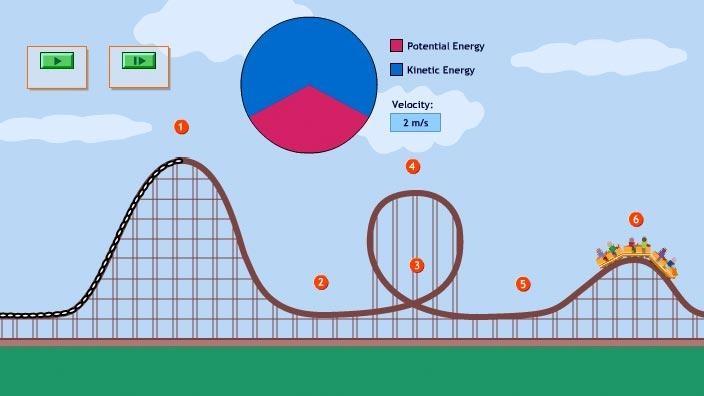
What number has the highest potential energy?
1
At what locations do you have your highest PE and Highest KE
PE:W
KE: Z
An irregularly shaped piece of silver was lowered into a graduated cylinder holding a volume of water equal to 25 ml. The height of the water rose to 40 ml. If the mass of the silver was 14 g, what was its density?
0.933g/ml 3
What is the period that the element cerium is found in ? and what is its atomic number?
period 6 and atomic number 58
What are the 4 things you find in the element box
Atomic mass, Atomic number, Chemical symbol, Element name

What number has the highest kinetic energy?
Both 2 and 5
Where is kinetic the highest and potential the highest
KE: 3
PE: 1 and 5


