Formula which shows the simplest whole-number ratio of atoms in a compound.
Empirical formula
This is the ability of carbon to form bonds with other carbon atoms (produces chains + ring structures)
Catenation
Which functional group does CH3Cl include?
Halogeno
Identify the functional group present in N-propylethanamide.
Amido group
IB Paper 1 Question!
Which of the following compounds contains an ester functional group?
A. CH3CH2COOCH
B. CH3CH2OCH3
C. CH3CH2COOH
D. CH3CONH2
A. CH3CH2COOCH
This is the shorthand representation of organic molecules, showing only its carbon backbone.
Skeletal Formula
A hydrocarbon chain with acyclic single bonds are… (saturated/unsaturated)
Unsaturated
Contains C=O which is present in Aldehydes and ketones
Carbonyl group
What functional group does 3-hexanone contain? (Be specific ;)
Carbonyl - Ketone
IB Paper 1 Question!
Which of the following compounds contains a carbonyl functional group?
A. Ethanol
B. Acetone
C. Ethanoic Acid
D. Propane-1-ol
B. Acetone
The formula C6H12O6 can be simplified to CH2O, by using this formula
Empirical formula
How can hydrocarbons with pi bonds become saturated?
Adding hydrogen molecules
Compounds containing this functional group often form weak acids.
Carboxyl group
Name the functional group present in 2, 2-dimethylbutane.
Alkyl group (unreactive carbon chain)
Name 2 differences and 1 similarity between aldehydes and ketones.
Similarity: contains carbonyl group
Differences:
Carbonyl group located in the terminal position (at one end of molecule) R-CHO (Aldehyde)
Carbonyl group attached to a C atom within the carbon chain R-CO-R' (Ketone)
Higher boiling point (Aldehyde)
More soluble in water (Aldehyde)
More reactive (Aldehyde)
Draw the skeletal formula of ethanol: CH3 - CH2 - OH

What three types of structures does the catenation of carbon atoms produce?
Straight chain, branched chain, ring structure
Which molecule has a carbonyl functional group?
A. CH3OCH3
B. CH3COCH3
C. CH3CH2OH
D. CH3CH2NH2
B. CH3COCH3
Here is the stereochemical formula for the sugar molecule glucose

What functional groups are present in glucose?
5 hydroxyl (alcohol)
1 carbonyl (aldehyde)
The amino and amido group differ in...
Amino group is composed of a nitrogen atom bonded to hydrogen atoms or alkyl groups.
Amido group is formed by a carbonyl group bonded to an amino group
Draw the full AND condensed structural formula of cyclohexane, C6H12

CHCHCHCHCHCH
Describe what a functional group does for their respective organic compounds.
Open-ended
Ex.
Gives compounds different properties
Determines the chemical reactivity of a molecule under a given set of conditions
WITHOUT looking at the handout, name 4 of the discussed functional groups, and write their functional group structure.
Open-ended
This is the skeletal formula of acetylsalicylic acid, otherwise known as aspirin.
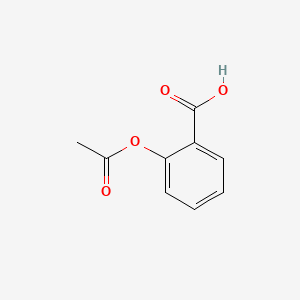
Phenyl group, carboxyl group, ester group
Here is the ball and stick model of diphenyl ether
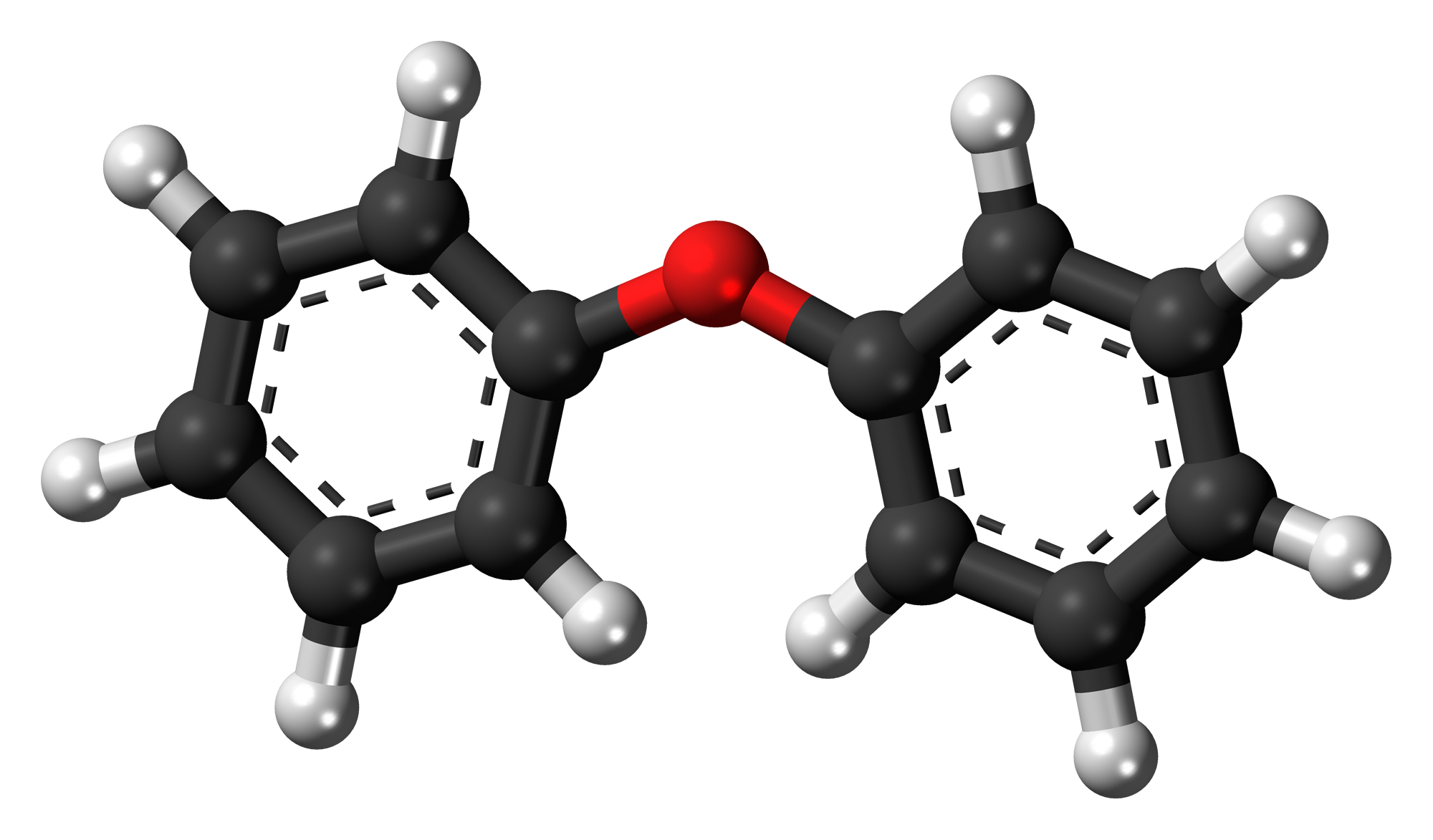
Determine the condensed structural formula for the compound and state the type of ether represented
(C6H5)2O or C6H5OC6H5
Aromatic ether