This type of diabetes results from autoimmune destruction of pancreatic β-cells and always requires insulin therapy.
What is Type 1 diabetes?
The recommended timing for GDM screening in average-risk pregnant women.
What is 24-28 weeks gestation?
The target HbA1c level women with pregestational diabetes should achieve before conception.
What is <6% (or as close to normal as possible without significant hypoglycemia)?
The length of time GLP1 should be discontinued prior to conception
What is 3 months?
The most common neonatal complication of maternal diabetes.
What is macrosomia (large for gestational age infant)?
The timing to begin antenatal testing in a well-controlled pregestational diabetic pregnancy.
What is 32 weeks gestation?
The HbA1c cutoff used to diagnose pregestational diabetes outside of pregnancy.
What is ≥6.5%?
These three fasting glucose values on a 3-hour OGTT would diagnose gestational diabetes (name at least 2 of 4).
What are ≥95 mg/dL (fasting), ≥180 mg/dL (1-hour), ≥155 mg/dL (2-hour), ≥140 mg/dL (3-hour)? [Note: need 2 or more abnormal values]
This medication, started at 4-5 mg daily preconceptionally, reduces neural tube defects in diabetic pregnancies.
What is folic acid?
The typical starting total daily insulin dose for a woman with pregestational diabetes in the first trimester.
What is 0.7 units/kg/day (based on pre-pregnancy weight)?
This serious maternal complication occurs when ketones accumulate in the blood, most commonly in Type 1 diabetics.
What is DKA?
The recommended delivery timing for a well-controlled GDM patient (A1) without other complications.
What is 39-40 weeks gestation?
GDM is classified into these two subtypes based on treatment requirements.
What are A1 GDM (diet-controlled) and A2 GDM (medication-requiring)?
Women with these risk factors should be screened for preexisting diabetes at their first prenatal visit.
What are obesity (BMI ≥30), prior GDM, strong family history of diabetes, or high-risk ethnicity (Hispanic, African American, Native American, Asian, Pacific Islander)?
Name the components of the comprehensive preconception or initial evaluation for a woman with pregestational diabetes in pregnancy.
What are HbA1c measurement, retinal examination, 24-hour urine for protein/creatinine clearance, serum creatinine, TSH, ECG (if long-standing diabetes or HTN), and medication review?
This oral hypoglycemic agent crosses the placenta, although minimally.
What is metformin?
Name three maternal complications that occur at higher rates in pregnancies complicated by diabetes.
What are preeclampsia, polyhydramnios, preterm birth, cesarean delivery, and postpartum hemorrhage?
The target blood glucose range during active labor for diabetic patients.
What is 72-126 mg/dL (or 80-120 mg/dL per some protocols)?
Explain the pathophysiologic differences between Type 1 and Type 2 diabetes and how this impacts insulin requirements during pregnancy.
Type 1 diabetes involves complete autoimmune destruction of β-cells leading to absolute insulin deficiency, requiring exogenous insulin from diagnosis and carrying higher risk of DKA. Type 2 diabetes involves peripheral insulin resistance with relative insulin deficiency, allowing for potential oral agent use initially, though most eventually need insulin in pregnancy. Type 1 patients have higher risk of microvascular complications (retinopathy, nephropathy) that can worsen in pregnancy, while Type 2 is more associated with obesity and metabolic syndrome complications.
Compare and contrast the one-step (75g OGTT) versus two-step (50g GCT → 100g OGTT) approach to GDM screening. Include diagnostic criteria for each and discuss the controversy around which method to use.
One-step: Single 75g OGTT at 24-28 weeks; diagnose if ≥1 abnormal value (fasting ≥92, 1-hr ≥180, 2-hr ≥153). Endorsed by ADA/IADPSG. Two-step: 50g GCT (if ≥140, proceed to 100g OGTT); diagnose if ≥2 abnormal values on 100g test (Carpenter-Coustan criteria). Endorsed by ACOG. Controversy: One-step identifies more GDM cases (~18% vs 9-10%), leading to more interventions and medicalization but potentially better outcomes. Two-step is more cost-effective, fewer patients need full testing, but may miss some at-risk patients. No consensus on superior approach; institutions choose based on resources and philosophy.
Explain the relationship between periconceptional glucose control and risk of congenital anomalies in diabetic pregnancies. Include specific HbA1c values and their associated risks, and discuss which organ systems are most commonly affected.
Congenital anomaly risk correlates directly with periconceptional HbA1c. HbA1c <6%: risk approaches baseline (2-3%). HbA1c 6-8%: 2-3× increased risk. HbA1c >8%: 4-5× increased risk (~10-15% anomaly rate). HbA1c >10%: risk may exceed 20%. Most critical period is 3-8 weeks post-conception (5-10 weeks gestation) during organogenesis. Most affected systems: cardiac (septal defects, conotruncal anomalies, cardiomyopathy), neural tube defects (anencephaly, spina bifida), caudal regression syndrome (specific to diabetes), renal anomalies, and skeletal defects.
Describe the physiologic changes in insulin requirements across pregnancy for a woman with pregestational diabetes. Include typical dosing at each trimester and explain the hormonal mechanisms driving these changes.
First trimester: Insulin requirements often DECREASE (0.7 units/kg/day) due to increased insulin sensitivity, nausea/vomiting reducing intake, and transfer of glucose to developing fetus. Risk of hypoglycemia highest.
Second trimester: Insulin requirements BEGIN to rise (0.8 units/kg/day) as placental hormones increase.
Third trimester: Insulin requirements increase dramatically (0.9-1.0+ units/kg/day, sometimes 1.2 units/kg/day) due to placental production of insulin-antagonistic hormones: human placental lactogen (hPL), progesterone, cortisol, and prolactin creating physiologic insulin resistance.
Post-delivery: Insulin requirements drop precipitously back to pre-pregnancy levels within 24-48 hours as placenta (hormone source) is delivered.
Explain the pathophysiology of fetal macrosomia in diabetic pregnancies and discuss why tight glucose control reduces this risk. Include the role of fetal hyperinsulinemia and describe the specific body composition changes seen in infants of diabetic mothers.
Pathophysiology: Maternal hyperglycemia → increased glucose crosses placenta → fetal hyperglycemia → fetal pancreatic β-cell hyperplasia → fetal hyperinsulinemia. Insulin acts as major fetal growth hormone in 2nd/3rd trimesters.
Hyperinsulinemia stimulates: increased protein synthesis, increased glycogen storage, increased lipogenesis (fat deposition). Result: macrosomia with specific pattern.
Body composition: Diabetic infants have asymmetric growth - increased shoulder-to-head ratio (organomegaly of liver, spleen, heart; increased subcutaneous and visceral fat) but relatively normal head circumference and length.
This increases shoulder dystocia risk. Abdominal circumference disproportionately large.
Tight control reduces macrosomia: evidence shows 50% reduction in macrosomia rates with optimal glucose control (fasting <95, 1-hr postprandial <140). Post-prandial control particularly important as post-meal spikes drive fetal insulin secretion.
Describe the typical regimen for starting a patient on a MDI insulin regimen
Discuss why GDM may actually represent undiagnosed preexisting Type 2 diabetes in many cases and explain the clinical implications of this distinction for postpartum management.
Many women diagnosed with GDM likely had undiagnosed Type 2 diabetes before pregnancy, especially with obesity epidemic and delayed childbearing. Evidence includes: persistently elevated HbA1c postpartum, need for medication immediately in pregnancy, and high fasting glucose values. Clinical implications include: need for postpartum glucose testing at 4-12 weeks (not just assuming resolution), potential need for continued medication postpartum, different counseling about future pregnancy risk (pregestational vs GDM recurrence), and earlier/more aggressive screening for overt diabetes in subsequent years. This affects long-term cardiovascular risk stratification and prevention strategies.
A 28-year-old G2P1 presents for her first prenatal visit at 10 weeks with BMI 42, prior 9 lb baby, and strong family history of Type 2 diabetes. Her random glucose today is 146 mg/dL. Walk through your diagnostic approach, explain what tests you would order and why, interpret possible results, and discuss how your management would differ if she has pregestational diabetes versus early GDM.
High-risk patient needs immediate testing for preexisting diabetes. Order fasting glucose or HbA1c (or both). Interpretation: HbA1c ≥6.5% or fasting glucose ≥126 = pregestational diabetes (likely Type 2). HbA1c 5.7-6.4% = prediabetes, rescreen at 24-28 weeks. Normal values = rescreen at 24-28 weeks. Management differences: Pregestational diabetes requires immediate tight control (goal HbA1c <6% if possible), comprehensive baseline evaluation (retinal exam, 24-hr urine protein, creatinine, TSH, ECG, detailed second-trimester ultrasound for anomalies, fetal echo, likely insulin from start, and higher-risk pregnancy classification.
A 30-year-old woman with Type 1 diabetes since age 12 presents for preconception counseling. Her current HbA1c is 8.1%, she's on MDI (NPH BID + regular insulin TID), had retinal photocoagulation 2 years ago, and wants to conceive "soon." Provide comprehensive preconception counseling including: risk assessment, required workup, medication optimization, and timeline for safe conception.
Risk assessment: Current HbA1c 8.1% confers ~10-15% risk of major congenital anomalies (vs 2-3% baseline). Prior retinopathy concerning - pregnancy can worsen. Must optimize before conception. Required workup: (1) Ophthalmology: updated retinal exam, may need additional photocoagulation before pregnancy; (2) Nephrology: 24-hr urine protein, creatinine clearance to assess kidney function; (3) Cardiology: ECG given duration of diabetes; (4) Labs: TSH (screen for autoimmune thyroid disease), update vaccines if needed. Medication optimization: Current regimen suboptimal. Options: (1) Switch to basal-bolus (glargine once daily + lispro/aspart with meals) for better control, or (2) Consider insulin pump if motivated. Start folic acid 4-5 mg daily now. Timeline: Need HbA1c <6% for at least 2-3 months before attempting conception to ensure stable control and reduce anomaly risk.
Compare and contrast insulin pump therapy versus multiple daily injections (MDI) for Type 1 diabetic pregnancies. Discuss patient selection criteria, advantages and disadvantages of each approach, troubleshooting considerations, and evidence for outcomes. Then explain how you would counsel a patient with Type 1 diabetes on MDI who asks about switching to a pump during pregnancy.
Pump vs MDI comparison: Pump advantages: More precise basal rate adjustments throughout day/night, easier to adjust for variable meal times, can temporarily reduce basal for exercise, potentially better overnight control, integration with CGM for hybrid closed-loop. Pump disadvantages: Requires high patient motivation/education, risk of DKA with catheter failure (no long-acting backup), mechanical failures, cost, steeper learning curve.
MDI advantages: Simpler, more familiar to providers, has long-acting insulin backup, less expensive, no mechanical failures, adequate control achievable. MDI disadvantages: Less flexible, multiple injections daily, harder to fine-tune, may have more hypoglycemia.
Patient selection: Pumps best for motivated patients with prior pump experience or difficulty with control on MDI, hypoglycemia unawareness, or significant dawn phenomenon. Evidence: Both can achieve excellent outcomes; pump may have slightly better HbA1c but not dramatically different perinatal outcomes.
Counseling for switch during pregnancy: Generally discourage switching TO pump during pregnancy unless preconception or early first trimester - takes 2-3 months to learn pump effectively, and pregnancy is not ideal time for learning curve. If patient insistent, would require: pump training before conception or very early pregnancy, close endocrinology partnership, frequent follow-up, and backup MDI plan. Better to optimize MDI for this pregnancy and transition to pump postpartum if desired for future pregnancies.
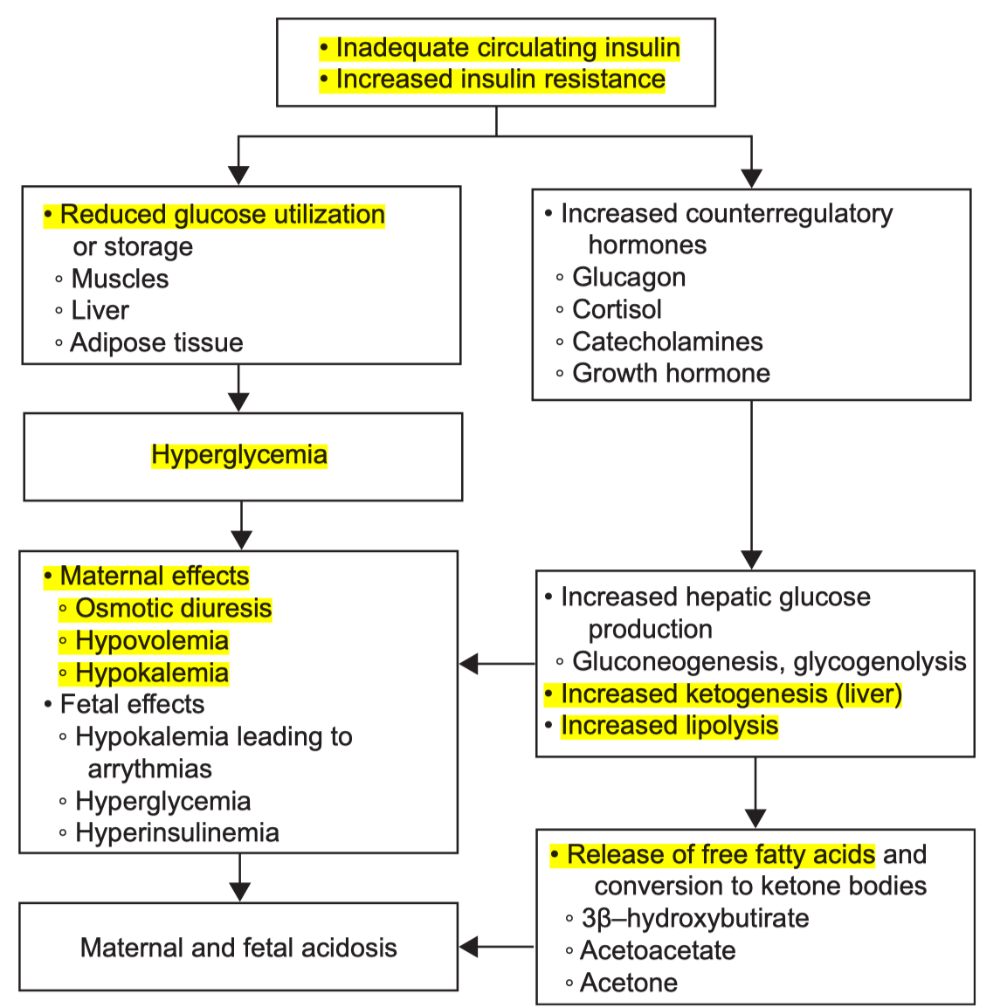
Explain the pathophysiology of DKA
The recommendations for insulin management for a DM1 (CGM, pump) presenting for scheduled IOL vs recommendations for DM2 presenting for scheduled IOL.
DM2: give standard basal insulin night before, hold morning basal (vs give 1/2), check q4 and q2
DM1: well controlled - continue pump in labor. Poor control - initially can do bolus, but low threshold to switch to insulin ggt/d5 infusion