(DO NOT PICK)
What is the smallest part into which an element can be divided and still maintain its properties?
What is an atom?
Jim rode his bike at a constant speed a half mile to school. Halfway to school, He stopped to buy a candy bar from the store. After buying the candy bar, Jim continued to ride his bike at a constant speed the rest of the way to school.
Which graph shows Jim's speed during his bike ride to school?
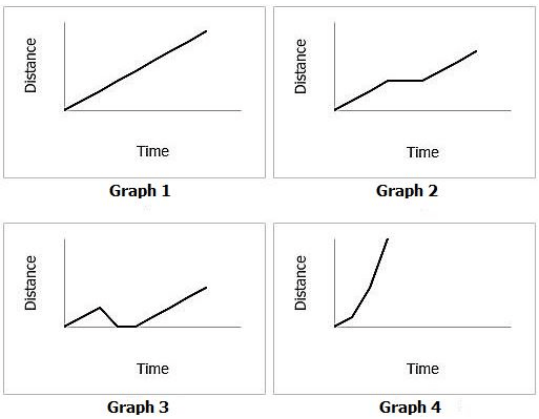
What is graph 2?
what is true about matter?
Matter is anything that has mass and takes up space.
In which beaker will salt dissolve the fastest?
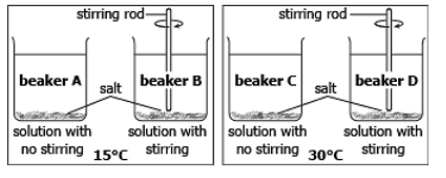
What is beaker D?
What action will stop an object is in motion?
what is force?
How many seeds can find in an average pumpkin?
When two or more substances combine to form a new substance, it it called a compound. Which substance is an example of a compound?
What is water?
What direction is the car on Shetland lane traveling? 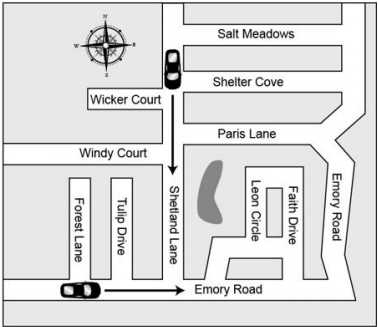
What is South?
A Styrofoam cup will float on the water's surface, but a penny will sink to the bottom. What physical property explains why the cup and the penny react the way they do in the water?
What is density?
What is the best way to separate a mixture of sand, salt, and water?
What is filtering and evaporating?
Which part(s) of these musical instruments possess(s) elastic potential energy?
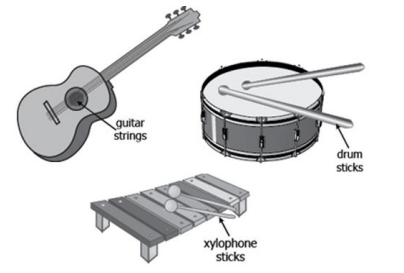
guitar stings only
What is the smallest physical unit of a substance that can exist independently?
What is a molecule?
A worker at a construction site pulls a cart filled with concrete blocks weighing 50kg each. He finds that the cart moves slowly.
What action would make the cart move fastest when pulled with the same force?
remove 10 concrete blocks
Which jar has the greatest mass? 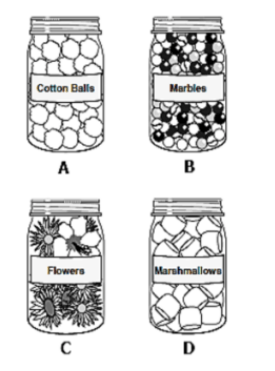
What is jar B?
A student adds water to a mixture of sand and sawdust. The sawdust rises with water, and the sand is left at the bottom.
What best describes the method used for separating the mixture?
What is flotation?
A teacher displays four images showing examples of potential energy.
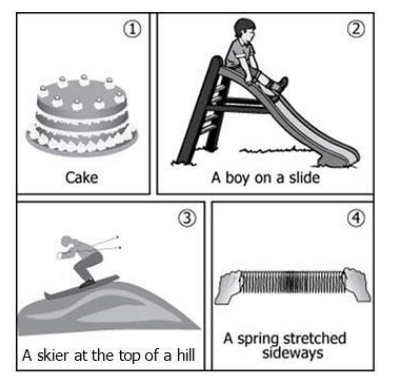 Which example(s) represent gravitational potential energy?
Which example(s) represent gravitational potential energy?
examples 2 and 3
When 2 oxygen atoms bond chemically with 2 hydrogen atoms, hydrogen peroxide is formed. Which phrase defines this substance?
What is a compound?
Which rock will require the most force to throw? 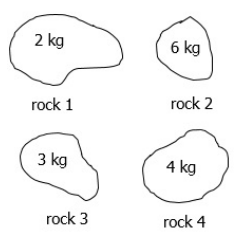
What is rock 2?
Which is true about gases and liquids?
No definite shape
As water evaporates, which best explains the change that is occurring?
A liquid changes to a gas.
Which object(s) possess(es) chemical potential energy?
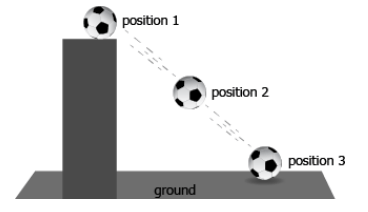
At which position(s) does the ball possess gravitational potential energy?
positions 1 and 2
Pure gasonline is made up of carbon and hydrogen. How are gasoline, hydrogen, and carbon classified?
Gasoline is a compound.
Hydrogen and carbon are elements.
Which term defines an object to which another object is compared?
What is a reference point?
What is the best way to classify E,F, and G. 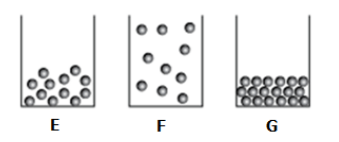
E: water
F: water vapor
G: Ice
Tracy wants to know which substance can be separated by filtration. She prepares four mixtures in four separate bowls.
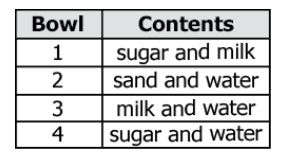 Which bowl's contents can be separated by filtration?
Which bowl's contents can be separated by filtration?
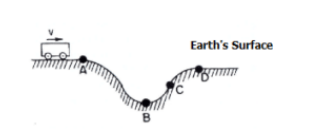 At which point on the track is the gravitational potential energy of the car the least.
At which point on the track is the gravitational potential energy of the car the least.
point B