The study of matter.
Chemistry
We use these as abbreviations for the element names on the periodic table.
Chemical Symbols
A group of atoms joined together.
Molecule
This measures the acidity or alkalinity of a substance.
Smallest piece of an element.
Atom
Substances containing only one kind of atom.
Element
Who is credited with creating the periodic table?
Dmitri Mendeleev
H2O and NaCl are examples of a ___.
Chemical Formula
What score would water recive on the pH scale?
7
These carry a positive charge.
Protons
A chemical change produces molecules known as __; they come from two or more different elements.
Compound
The vertical columns on the periodic table are called __.
Groups
The number of protons in the nucleus of an atom also tells us an elements ____.
Atomic Number
A solution with a score of 6.3 on the pH scale is considered a(n) ___.
Acid
What is the maximum number of electrons an atom can have in its first shell?
Two
Anything that has mass and takes up space.
How many protons are found in the following element?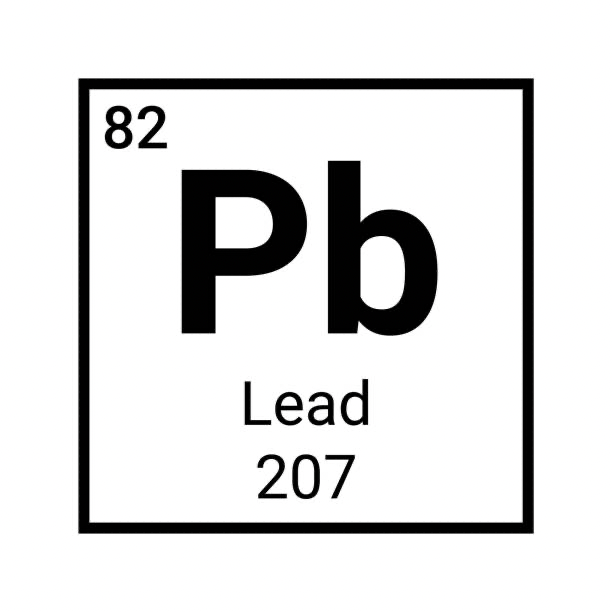
82
Who is responsible for first studying atomic structure?
Niels Bohr
Bleach is considered a __ with a pH score of 13.
Base
Another name for chemical symbols is ___.
Atomic Symbols
An atom that has gained or lost electrons.
Ion
Based on the information provided, what is the atomic mass of the element platinum?
195.08
How many carbon atoms are in lactic acid: C3H6O3
Three
Acids form what kind of ions?
Hydrogen Ions (H+)
What two things make up an atoms nucleus?
Protons and Neutrons