The three states of matter.
What is solids, liquids, and gases?
Protons and neutrons are located in this part of the atom.
What is the nucleus?
One of a hundred of substances that cannot be broken down into smaller parts.
What is an element?
This is how you find density.
What is divide the mass by the volume?
Mass is ___________________________.
What is the amount of matter in an object?
In this state, both shape and volume take the shape of the container.
What is gas?
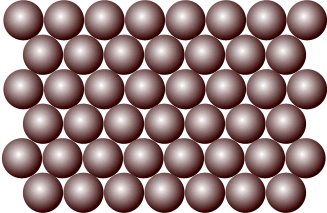
What is a solid?
These contain a negative charge and circle the nucleus of an atom.
What are electrons?
The number of different elements in O=H=O.
What is two?
Which has a greater mass, a metric ton of feathers or a metric ton of bricks?
What is neither, they have exactly the same mass: one metric ton.
Density is ______________________
What is certain amount of matter in a certain amount of space?
This is the amount of matter a substance contains.
What is mass?
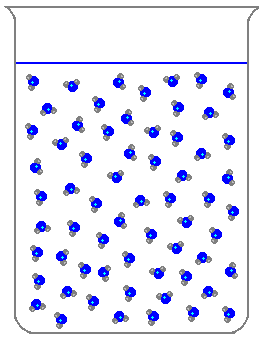
What is a liquid?
The number of electrons in this atom.
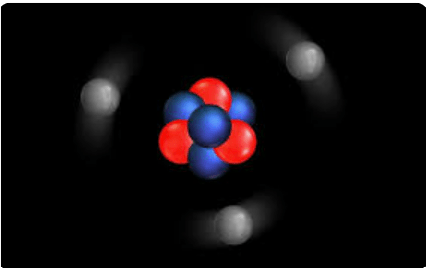
What is three?
Salt (NaCl), water (H20), and iron sulfide (FeS) are examples of this.
What are molecules?
Two blocks have the same mass- 10 grams
Block A has a volume of 2 ml
Block B has a volume of 5 ml
Block ________ has the greater density.
What is Block A?
How much space an object takes up.
What is volume?
The density of an object that has a mass of 10 grams and a volume of 5 milliliters.
What is 2 grams/milliliter?
When a substance changes from a solid to a liquid.
What is melting?
The number of electrons in this atom.
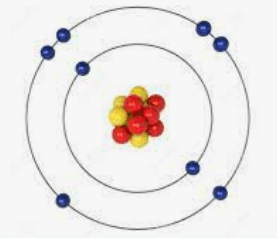
What is 8?
There are over 100 of these organized on the periodic chart of _________________.
What is elements?
Rhonda wanted to measure the volume of a rock. She measured out a volume of 100 ml of water. Then she put the rock in the graduated cylinder. The water now measured 123 ml.
_________ is the volume of the rock.
What is 23 ml?
This ball has greater density.
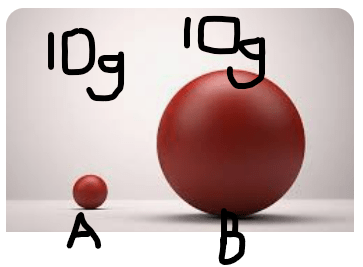
What is ball A?
What is sink?
Particles are very far apart and moving very quickly in a _________________.
What is gas?
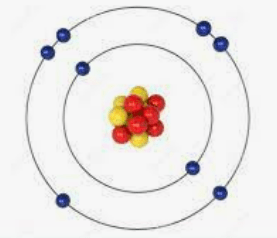
How many protons does this atom have?
Hint: The protons = electrons.
What is 6?
When you add the number of protons to the number of neutrons you get the ________________.
What is atomic mass?
The quarter in my pocket sinks in a cup of water. The density of the quarter must be greater than 1 g/ml or less than t1 g/ml.
What is greater than 1 g/ml?
Two blocks that have the same volume but have different densities have __________________.
What is different masses?
An object with a density of 10 g/ml has a mass of 100 grams. This is its volume.
What is 10 milliliters?
When a solid changes directly from a solid to a gas skipping the liquid phase.
What is sublimation?
When you add the number of protons to the number of neutrons you get the ___________.
What is the atomic mass?
__________ determines what an element is.
What are the number of protons?
An object will sink in water has a density ________ than the density of water.
What is greater?
Everything is made of this.
What is matter?
Carbon dioxide put in water will go from a solid directly to a gas. This is what that is called.
What is sublimation?