Define an acid
a substance that can donate a hydrogen ion or proton
Define a bas
a substance that can accept a hydrogen ion or proton
They can neutralize each other, making a salt and water.
What is an inorganic compound?
It is a compound without carbon or a carbon containing compound like carbon dioxide.
A compound based in carbon. Can have oxygen or hydrogen. May have nitrogen, sulfur or phosphorous.
Give an example of an acid
HCl, acetic acid, HF etc.
Give an example of an acid
NaOH, soap, ammonia
How is the strength of an acid or a base measured?
It is measured by how much of the acid or base dissolves in water. If the substance fully dissolves, it is a strong acid/base.
What type of bonds are possible between carbons?
Single,double, triple
What is the difference between a straight chain and a branched chain?
In a straight chain, all the carbons (except those on the ends) are attached to two carbons.
In a branched chain, there is one or more carbon that is attached to three carbons or four carbons.
What pH do acids have?
The pH is between 0 and 6.
What pH do bases have?
The pH of bases is between 8 and 14.
How many electrons are shared in a double bond?
Please draw a full structural formula on the board.

How many bonds can carbon make?
4 bonds
What happens when solid acids dissolve in water? Be specific!
They break up into ions, H+ and an anion
What happens when solid acids dissolve in water? Be specific!
They react with water and break up into ions, OH- and a cation.
Draw a benzene ring on the board
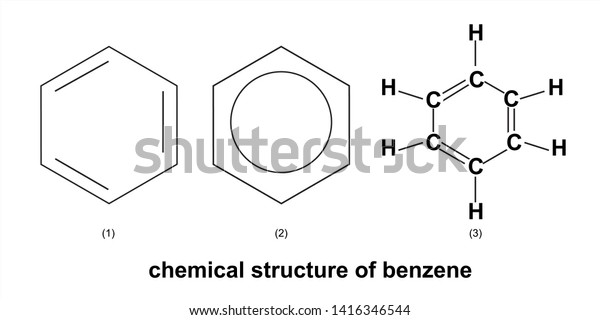
Name 2 different forms of carbon only compounds
Graphite, diamonds, bucky ball, carbon nanotubes
How many electrons are shared in a single bond?
2 electrons
Name 3 characteristics of acids
They are sour in food, they produce a burning or prickling feeling, they produce hydrogen gas when they react with metal, they turn litmus paper red
Name 3 characteristics of bases.
have a slippery feeling, may taste bitter in foods, react with fatty acids, will turn litmus paper blue.
What are compounds with benzene rings also called?
Aromatics
Give an example of a compound with a benzene ring
Vanillin, polystyrene
Explain what an isomer is
Compounds that contain the same atoms but in different places/arrangements