Elements are atoms that have different __________
Properties or Neutron/Proton/Electrons
________ Is the ability to do work (cause change)
Force
A combination of salt and sand is known as a ___________. Which can be easily seperated
Mixture
Chemical
Which state of matter takes the space of the container its in?
Gas
Which element is most similar to Nitrogen (N)?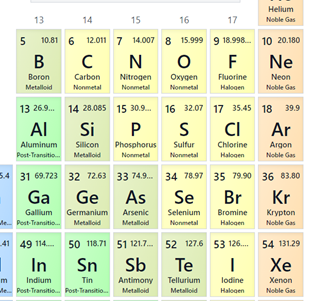
Phosphorous (P)
________ Force is capable of acting at a distance, but only with ferrous (iron based) materials.
Magnetic
Atoms are made of three subatomic particles, what are the two found in the nucleus (center) of the atom?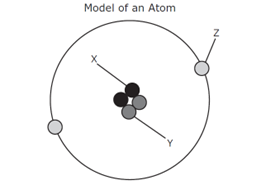
Neutron and Proton
pH goes from 0 to 14, with 7 being Neutral. Which range of numbers relates to basic solutions.
8 to 14
Which states of matter have no definite shape?
Liquid and Gas
_________ Is the name of the horizontal rows that represent different electron levels.
Period
Gravity can affect the weight of an object based on _________ and __________
Mass and distance
What is the name and charge of subatomic particle Z?
Electron and Negative
pH goes from 0 to 14, with 7 being Neutral. Which range of numbers relates to acidic solutions.
0 to 6
What are the names of the following transitions? Solid to liquid & Liquid to Gas?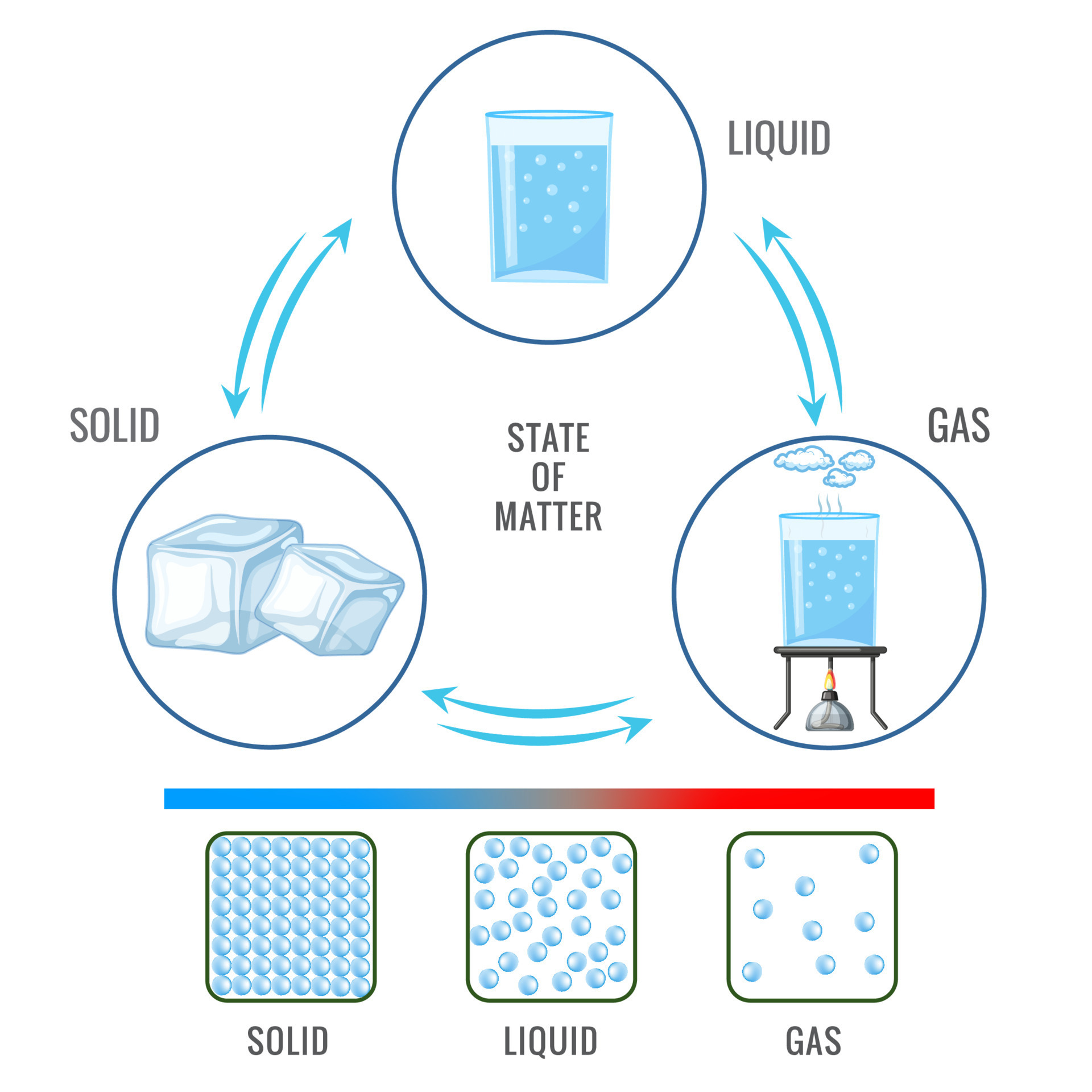
Melting, Evaporation
_________ Is the vertical columns from that show elements with similar properties.
Groups
As a rocket exits Earth's atmosphere and gravitational pull, what changes with its mass and weight?
Mass remains the same, weight is changed
What is the measure of the amount of space an object takes up?
Volume
7
________ have the highest kinetic energy and ______ has the highest potential energy
Gas, Solid
The category of elements that are shiny, conductive and malleable are known as _________
Metals
What is the unit used to measure force?
Newton (N)
When an object sinks in a liquid, what are the relative densities (which one is less dense and which one is higher?)
Liquid is less dense, Object is more dense
D = m/v, which object has the HIGHEST density?
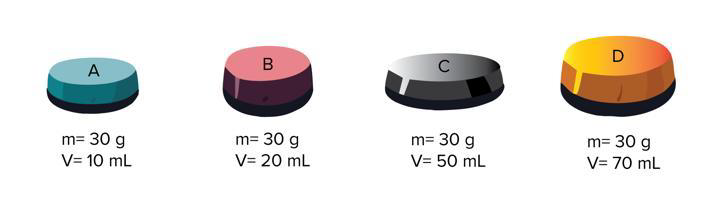
A
DOUBLE POINTS: What phase change is from solid directly to gas?
Sublimation