Anything that has mass and takes up space
What is Matter?
The three states of matter
What is solid, liquid and gas?
A Chemist's most important tool in learning the behavior and properties of elements
What is the Periodic Table?
The center of the atom
What is nucleus?
A Covalent Bond
What is when atoms share electrons?
H2 + O2 --> H2O
What is 2H2 + O2 --> 2H2O
The two factors responsible for phase changes in matter
What is temperature and pressure?
A simplified model of the atom
What is the Bohr Model?
This mineral is essential for thyroid hormones and weighs in at 126.9u
What is Iodine?
An atom that loses an electron
What is a cation?
Formed when ions are attracted to each other
What is Ionic Bond?
Ag2O --> Ag + O2
What is 2Ag2O --> 4Ag + O2
The subatomic particles
What is proton, neutron and electron?
What is the outermost level/shell of electrons?
Has 4 shells and 5 valence electrons
What is Arsenic?
An atom that gains an electron
What is an anion?
In sweet tea, sugar is this part of the solution
What is a solute?
CO2 + H2O --> C6H12O6 + O2
What is 6 CO2 + 6 H2O --> C6H12O6 + 6 O2
Two or more atoms linked together to make a substance with unique properties
What is a molecule?
The only subatomic particle that can move from the atom
What is an electron?
Define the Groups and Periods
What is columns are groups that indicate how many valence electrons an element has. The rows are Periods and indicate how many electron shells/energy levels an element has?
This subatomic particle is about 2,000 times smaller than it's counterparts.
What is Electron?
AB + C --> B + AC
What is a Single Replacement Reaction?
FeCl3 + NaOH --> Fe(OH)3 + NaCl
What is FeCl3 + 3 NaOH --> Fe(OH)3 + 3 NaCl
One or more elements that are chemically bonded together
What is a Compound?
The sum of the atomic number and the number of neutrons
What is the atomic mass?
The Atomic Number of Oxygen is 8, what would happen if I added one more proton to it's nucleus?
What is you would no longer have Oxygen? You'd have Fluorine.
Most matter on Earth has an overall charge of
What is Neutral?
Exchanges in two compounds to form two completely new compounds, with the exchange of ions between the reactants.
What is a Double Replacement Reaction?
S8 +O2 --> SO3
What is S8 +12 O2 --> 8 SO3
New substances formed as a result of a chemical change
What is a Product?
The types of elements located on the left side of the periodic table
What is Metals?
Helium only has 2 valence electrons but is located in Group 8
What is Helium only has one shell so the max valence electrons it can have is 2. It has a full outer shell and meets the requirement of being non-reactive like the other Noble Gases?
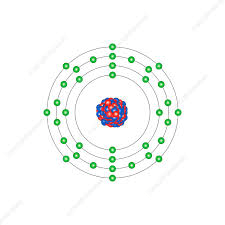
What is Krypton?
Name this bond
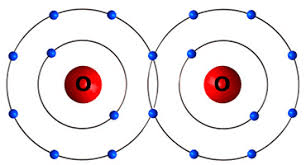
What is a Covalent Bond?
Fe2O3 + C ---> Fe + CO2
What is 2Fe2O3 + 3C ---> 4Fe + 3CO2